Tricyclic compounds and pharmaceutical uses thereof
A compound and pharmaceutical technology, applied in the field of tricyclic compounds and their pharmaceutical uses, can solve problems such as poor clinical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
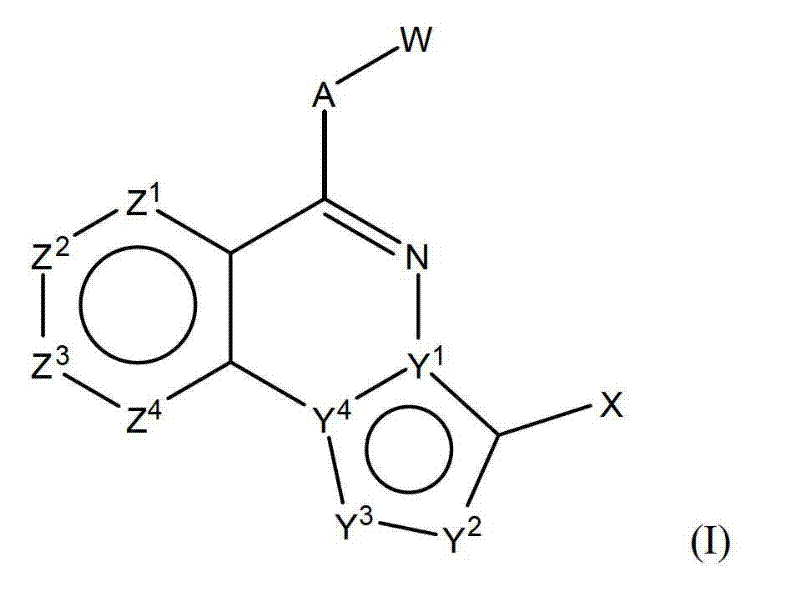
[0140] The present invention provides compounds of formula (I):
[0141]
[0142] in:
[0143] Z 1 ,Z 2 ,Z 3 and Z 4 Each independently is CR 1 or N if Z 1 to Z 4 At most two of are N and contain Z 1 to Z 4 The ring of is aromatic;
[0144] Each R 1 independently H, halo, CN, optionally substituted C1-C4 alkyl, optionally substituted C2-C4 alkenyl, optionally substituted C2-C4 alkynyl, optionally substituted C1-C4 alkoxy , SR, SO 2 R, COOR, COONR 7 R 8 or -NR 7 R 8 ;
[0145] Y 1 and Y 4 Each is C or N, and Y 1 and Y 4 Not both are N at the same time; the condition is Y 1 to Y 4 At least one of is N;
[0146] Y 2 Yes N, NR 2 、CR 2 or CX 2 ,
[0147] where each R 2 are independently H, -OR, halo, CN or optionally substituted C1-C4 alkyl,
[0148] where each R 2 Yes-(CH 2 ) 0-2 COOR or polar groups;
[0149] X is -(CH 2 ) 0-2 COOR or polar group, or when Y 2 is CX 2 when X is R 2 ;
[0150] each R is independently H or an optionally sub...
Embodiment
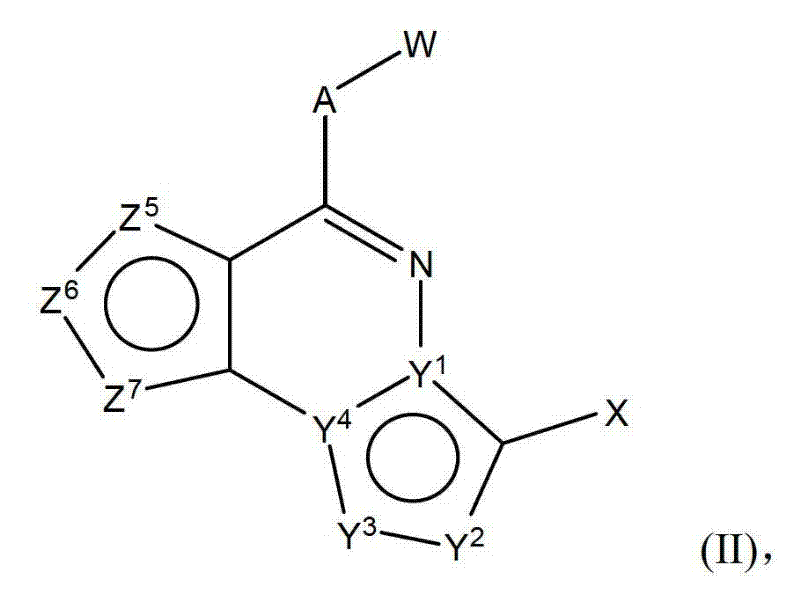
[0299]The following examples illustrate but do not limit the invention. In particular embodiments, the invention includes any compound of formula (I) or formula (II) described in the reaction schemes and examples. A variety of compounds of formula (I) and / or II can be readily prepared by one of ordinary skill by using the following general schemes, together with knowledge and reagents available in the art. It is to be understood that said compounds are within said formula (I) or (II), even if the atomic symbols used in the schemes are different from those used in formulas (I) and (II).
[0300] Reaction scheme 1
[0301] Certain compounds of formula (I) can be prepared by the general methods illustrated in Scheme 1 . Nitrile (3) can be deprotonated by (a) using a base such as but not limited to N-butyllithium, then (b) contacting the anion obtained from step (a) with acid chloride (2) or ester (1) to Compound (4) is provided, whereby a compound of formula (8) is obtained. ...
Embodiment 7
[0326] Compounds of formula (II), such as compounds (8) in Scheme 7, can be prepared using the transformations described in Scheme 3, as described below.
[0327] Option 7
[0328]
[0329] Reaction scheme 8
[0330] Compounds of formula (II), such as compounds (8) in Scheme 8, can be prepared using the transformations described in Scheme 3, as described below.
[0331] Option 8
[0332]
[0333] Reaction scheme 9
[0334] Compounds of formula (II), such as compounds (8) in Scheme 9, can be prepared using the transformations described in Scheme 3, as described below.
[0335] Option 9
[0336]
[0337] Reaction scheme 10
[0338] Compounds of formula (I), such as compound (3) in scheme 10, can be prepared as described in Journal fuer Praktische Chemie 1981, 323, 647-653. Compound (8) can be prepared from compound (3) as described in Scheme 3.
[0339] Scheme 10
[0340]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com