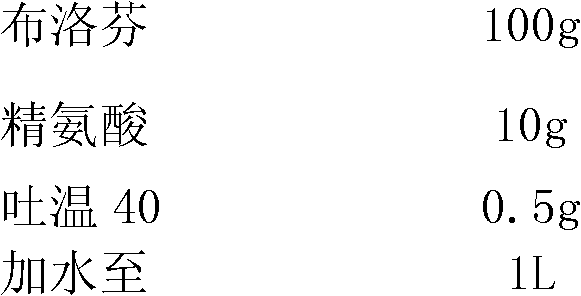Water-soluble ibuprofen pharmaceutical composition
A composition and drug technology, applied in the field of medicine, can solve the problems of poor water solubility of ibuprofen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] Take 500ml of water for injection, add arginine and ibuprofen, stir until dissolved, then add Tween 40, stir to dissolve and mix evenly, add 0.2% (2g / L) medicinal activated carbon, stir for 30min, filter to remove medicinal carbon, cooled to room temperature, then added water to 1 L, and finely filtered through a 0.22 μm microporous membrane to obtain a solution of the composition of the present invention with a concentration of 100 mg / mL.
[0025] The ibuprofen pharmaceutical composition solution is packaged in ampoules, sterilized at 121° C. for 15 minutes, packaged and then stored, and divided into 1000 bottles. In clinical use, it is diluted to the required concentration with physiological saline or glucose solution.
Embodiment 2
[0027]
[0028]
[0029] Take 2.5L of water for injection, add arginine and ibuprofen, stir until dissolved, then add Tween 60, stir to dissolve and mix evenly, add 0.2% medicinal activated carbon, stir for 30min, filter to remove medicinal carbon, and then continue Add water for injection to dilute to 5 L, fine filter with a 0.22 μm microporous membrane, and obtain the composition solution of the present invention. The concentration is 100mg / mL.
[0030] Injection was prepared according to the method of Example 1.
Embodiment 3
[0032]
[0033]Take 3L of water for injection, add arginine, ibuprofen, Tween 80, stir until dissolved, then add propylene glycol, stir to mix evenly, add 0.2% (2g / L) medicinal activated carbon, stir for 30min, filter to remove drug Dilute to 5 L with carbon, and then continue to add water for injection, fine filter with a 0.22 μm microporous membrane, and obtain the solution of the composition of the present invention. The concentration is 100mg / mL.
[0034] Injection was prepared according to the method of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com