Mixture for non-aqueous electrolyte secondary battery, electrode for same, and non-aqueous electrolyte secondary battery
A non-aqueous electrolyte, secondary battery technology, applied in non-aqueous electrolyte battery electrodes, battery electrodes, circuits, etc., can solve the problem of insufficient electrode peel strength, and achieve excellent peel strength, suppression of uneven existence, and good productivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0113] [Manufacture Example 1] (Manufacture of carboxyl group-containing vinylidene fluoride polymer (1))
[0114] 1040g of ion-exchanged water, 0.8g of methyl cellulose, 3.0g of diisopropyl peroxydicarbonate, 396g of vinylidene fluoride and 4.0g of monomethyl maleate were charged into an autoclave with an inner capacity of 2 liters. The suspension polymerization was carried out at 45°C for 45 hours. The highest pressure during this period reached 4.1MPa. After the polymerization is completed, the polymer slurry is dehydrated and washed with water. Thereafter, drying was performed at 80° C. for 20 hours to obtain a powdery carboxyl group-containing vinylidene fluoride polymer (1) (polymer (1)). The weight-average molecular weight of polymer (1) is 500,000, and inherent viscosity is 1.7dl / g, I R (=I 1650-1800 / I 3000-3100 )(It should be noted that at 1750cm -1 The absorbance from the carbonyl group was observed at 3025 cm -1 The absorbance from the CH structure was obser...
manufacture example 2
[0115] [Manufacture example 2] (manufacture of polyvinylidene fluoride)
[0116] 1075g of ion-exchanged water, 0.4g of methyl cellulose, 2.5g of di-n-propyl peroxydicarbonate, 5g of ethyl acetate, and 420g of vinylidene fluoride were charged into an autoclave with an inner capacity of 2 liters, and carried out at 25°C for 14 hours of suspension polymerization. The highest pressure during this period reached 4.0MPa. After the polymerization is completed, the polymer slurry is dehydrated and washed with water. Thereafter, drying was performed at 80° C. for 20 hours to obtain powdery polyvinylidene fluoride (PVDF). The weight average molecular weight of PVDF is 500,000, and the logarithmic viscosity is 1.7dl / g.
[0117] [Measurement of weight-average molecular weight of carboxyl group-containing vinylidene fluoride polymer (1) and polyvinylidene fluoride]
[0118] The polystyrene-equivalent weight average molecular weights of the carboxyl group-containing vinylidene fluoride ...
Embodiment 1
[0132] (Preparation of mixture for non-aqueous electrolyte secondary battery)
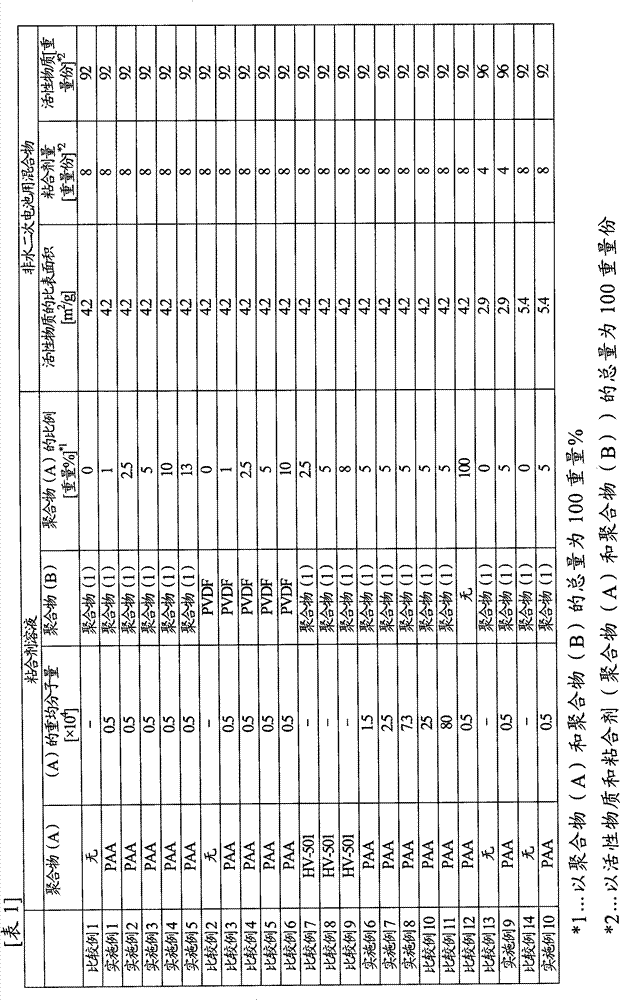
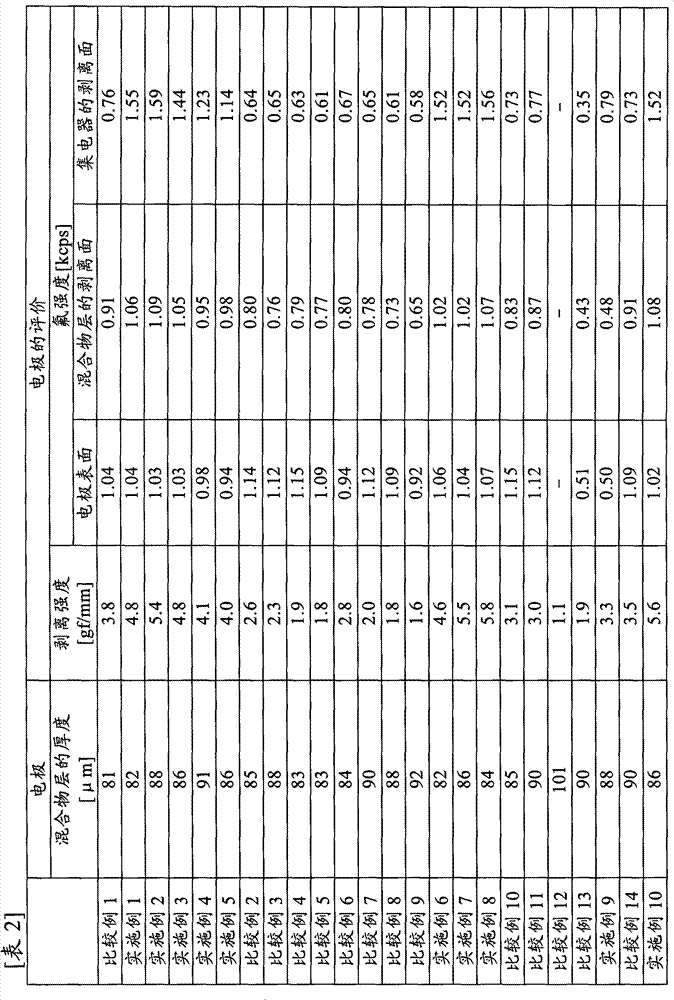
[0133] Polyacrylic acid (manufactured by Wako Pure Chemical Industries, Ltd., carboxyl group content: 1.4 × 10 -2 mol / g) 0.1 g was dissolved in 90 g of N-methyl-2-pyrrolidone to obtain a 10% by weight binder solution (1). 8 g of the obtained binder solution (1), artificial graphite (manufactured by Hitachi Chemical Industry, MAG, average particle diameter 20 μm, specific surface area 4.2 m 2 / g) 9.2 g and 5.8 g of N-methyl-2-pyrrolidone for adjusting the viscosity of the mixture were stirred and mixed to obtain a mixture (1) for a nonaqueous electrolyte secondary battery. The viscosity of the mixture (1) for nonaqueous electrolyte secondary batteries was 12000 mPa·s.
[0134] (production of electrodes)
[0135] The obtained mixture (1) for a non-aqueous electrolyte secondary battery was coated on a copper foil having a thickness of 10 μm as a current collector using a separator and a bar coater ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com