Derivatives of vinylidene fluoride as components of liquid crystal medium and their preparation methods and applications
A technology of reaction and compound, which is applied in the field of difluoroethylene derivatives as components of liquid crystal media and its preparation and application, and can solve problems such as weak negative dielectric anisotropy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
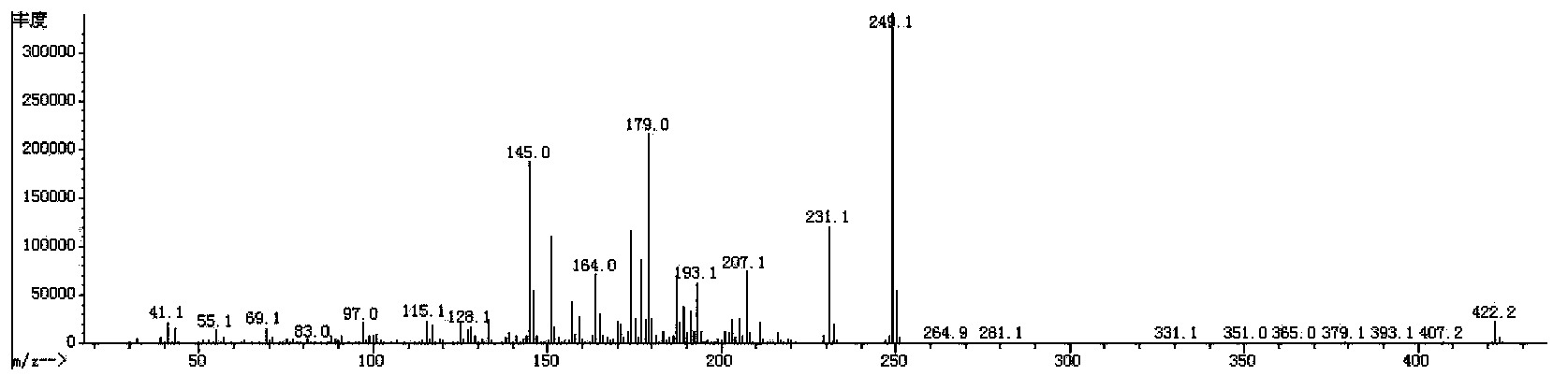
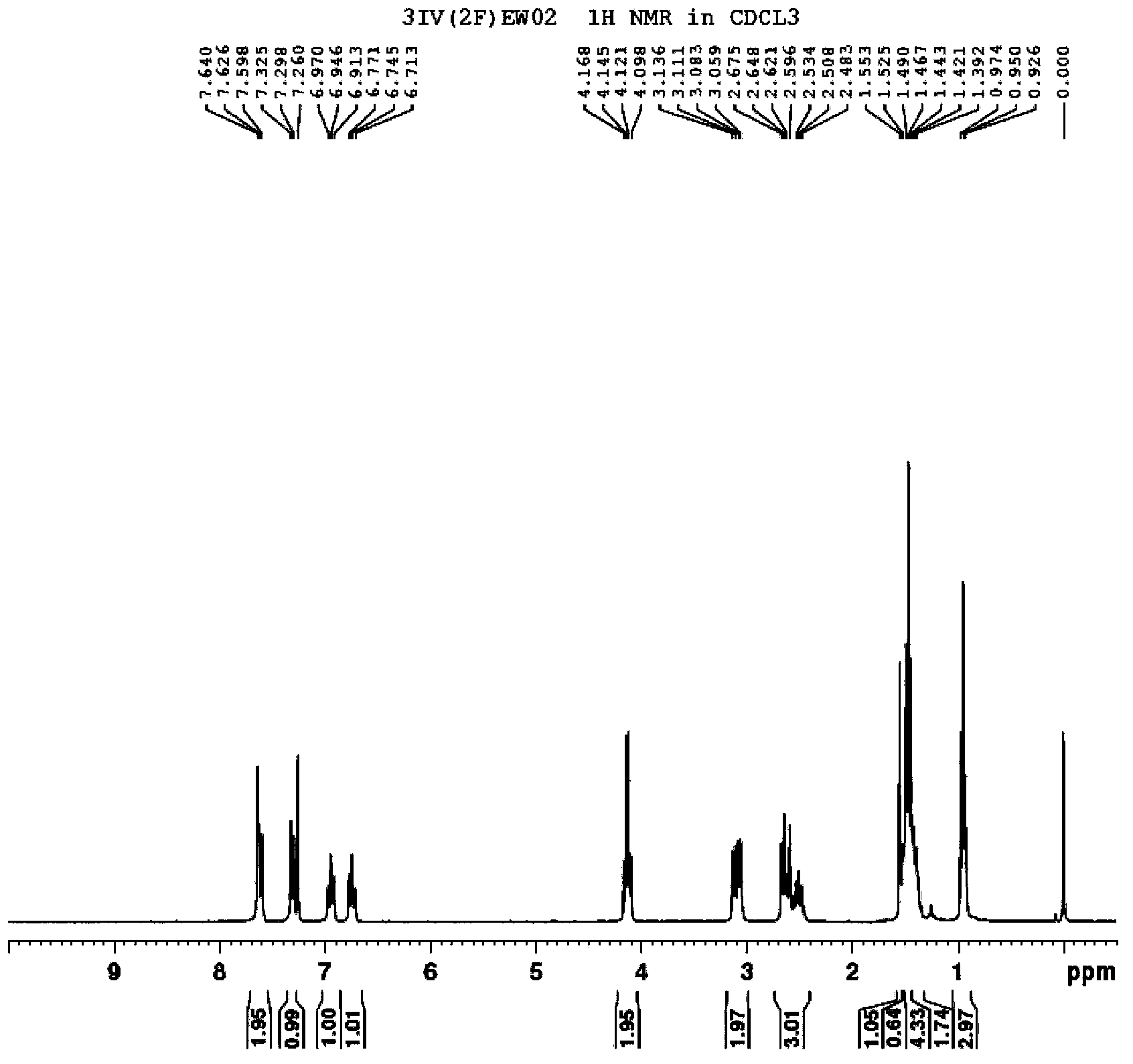
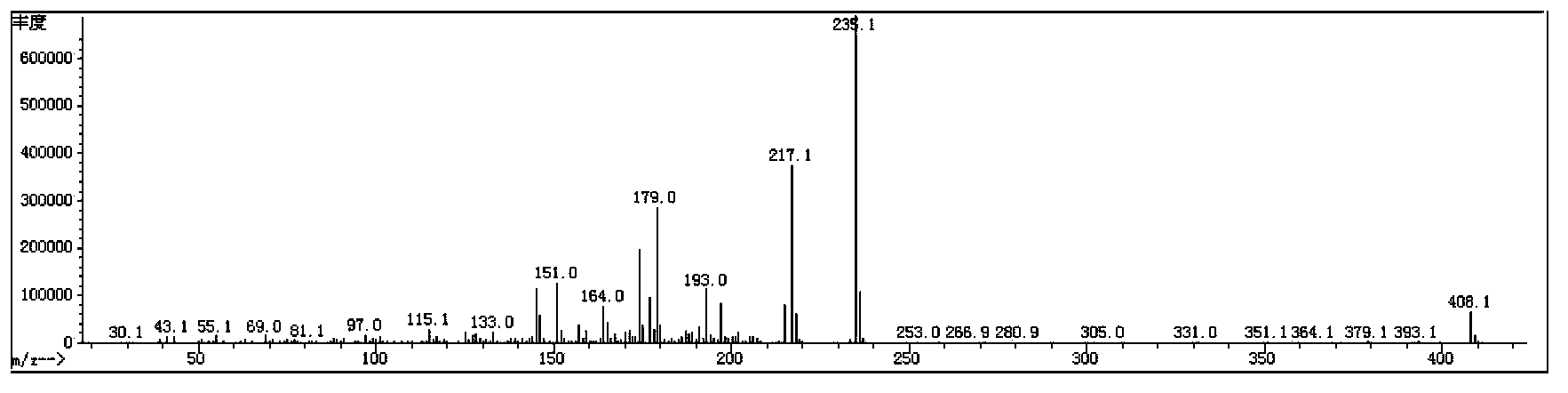
[0112] The synthetic route of the prepared compound 3IV (2F) EWO2 is as follows:
[0113]
[0114] Its specific process steps are as follows:
[0115] 1) Synthesis of 3IV(2F)EWO2-2
[0116] Add 5.76g of Mg powder and 0.05g of iodine into a 500mL three-necked flask, 10mL of tetrahydrofuran (THF), and protect with nitrogen. Dissolve 47.4g of 3IV(2F)EWO2-1 (commercially available intermediate) compound in 200mL tetrahydrofuran solution, slowly add the tetrahydrofuran solution of 3IV(2F)EWO2-1 compound to the above reaction system, and wait for the reaction to start , continue to dropwise add the THF solution of the remaining 3IV(2F)EWO2-1 compound, after the dropwise completion, keep the system under slight boiling and reflux for 3h, and cool to room temperature. 40g of chlorotrifluoroethylene was slowly passed into the cooled reaction system, and stirred at room temperature for 12h. The reaction was quenched with dilute hydrochloric acid, extracted with ethyl acetate, the ...
Embodiment 2
[0133] The synthetic route of the prepared compound 2IV (2F) EPWO is as follows:
[0134]
[0135] Its specific process steps are as follows:
[0136] 1) Synthesis of 2IV(2F)EPWO2-9
[0137] Add 5.76g of Mg powder and 0.05g of iodine into a 500mL three-necked flask, 10mL of tetrahydrofuran (THF), and protect with nitrogen. Dissolve 44.6g of 2IV(2F)EPWO2-8 (commercially available intermediate) compound in 200mL tetrahydrofuran solution, slowly add the tetrahydrofuran solution of 2IV(2F)EPWO2-8 compound to the above reaction system, and wait for the reaction to start , continue to add dropwise the remaining THF solution of the compound of 2IV(2F)EPWO2-8, after dropping, keep the system slightly boiling and reflux for 3h, and cool to room temperature. 40g of chlorotrifluoroethylene was slowly passed into the cooled reaction system, and stirred at room temperature for 12h. The reaction was quenched with dilute hydrochloric acid, extracted with ethyl acetate, the organic phas...
Embodiment 3
[0153] The synthetic route of the prepared compound 2IV (2F) EWP3 is as follows:
[0154]
[0155] Its specific process steps are as follows:
[0156] 1) Synthesis of 2IV(2F)EWP3-9
[0157] Add 5.76g of Mg powder and 0.05g of iodine into a 500mL three-necked flask, 10mL of tetrahydrofuran (THF), and protect with nitrogen. Dissolve 44.6g of the 2IV(2F)EWP3-8 (commercially available intermediate) compound in 200mL THF solution, slowly add the THF solution of the 2IV(2F)EWP3-8 compound to the above reaction system, and wait for the reaction to start , continue to dropwise add the remaining THF solution of the compound of 2IV(2F)EWP3-8, after the dropwise completion, keep the system under slight boiling and reflux for 3h, and cool to room temperature. 40g of chlorotrifluoroethylene was slowly passed into the cooled reaction system, and stirred at room temperature for 12h. The reaction was quenched with dilute hydrochloric acid, extracted with ethyl acetate, the organic phase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com