Methods for performing a coronary artery bypass graft procedure
A technology for coronary artery and coronary artery disease, which can be applied to surgical drugs, devices with human tubular structures, and pharmaceutical formulations, etc., and can solve problems such as resource consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
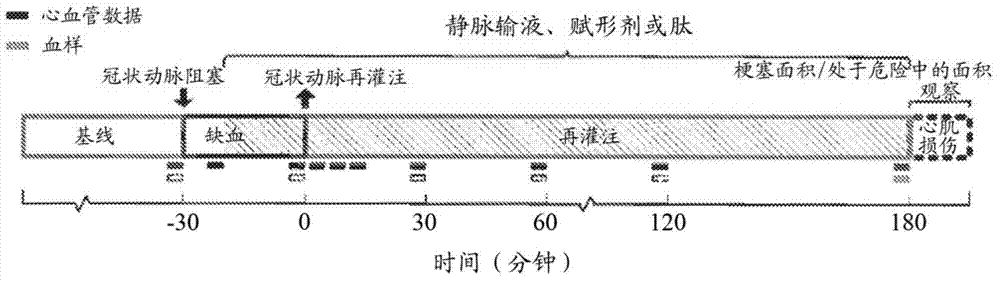
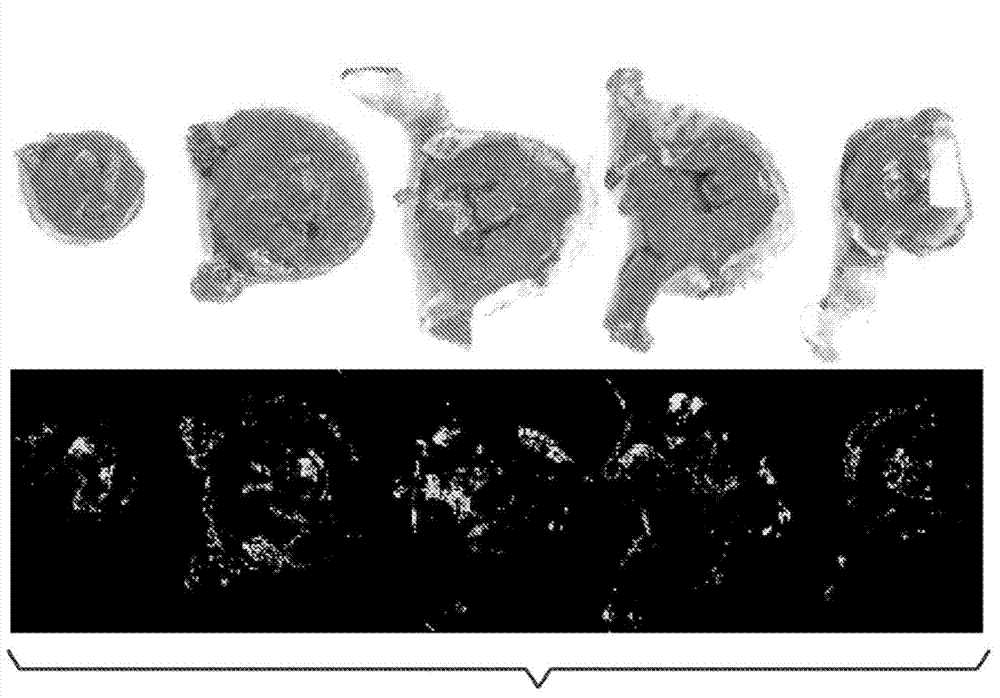
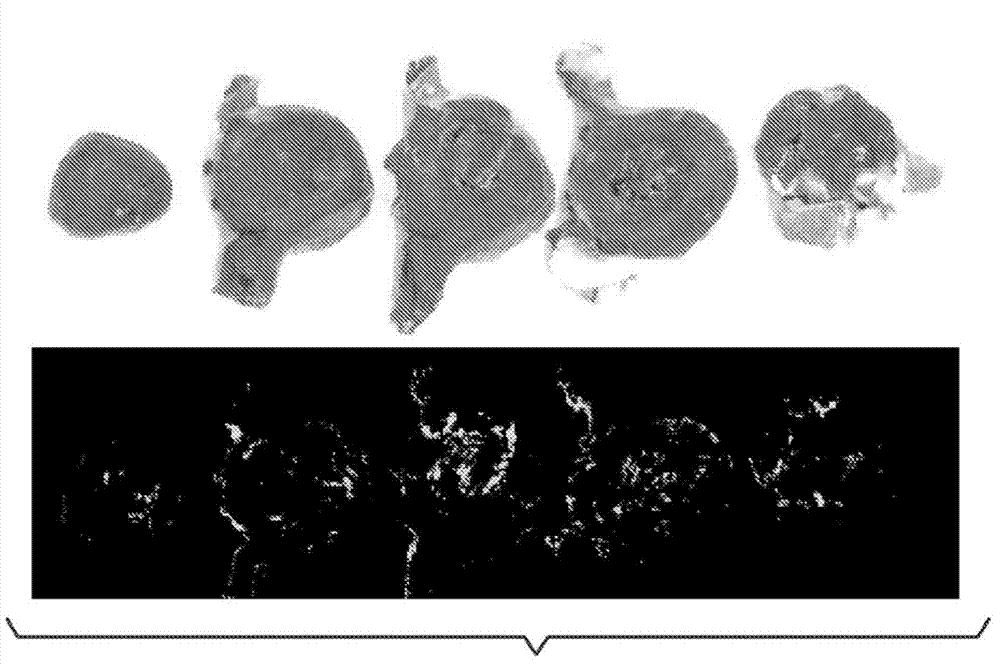
[0246] Example 1. The role of aromatic-cationic peptides in protecting rabbit models from vascular occlusion injury
[0247] The role of aromatic-cationic peptides in protecting rabbit models from vascular occlusive injury was investigated. Peptide D-Arg-2'6'-Dmt-Lys-Phe-NH is confirmed by this example 2 cardioprotective effect.
[0248] experimental method
[0249] New Zealand white rabbits were used in this study. The rabbit is male and older than 10 weeks. The environmental controls of the animal room were set to maintain a temperature of 61°F to 72°F and a relative humidity between 30% and 70%. Room temperature and humidity were recorded hourly and monitored daily. There were about 10-15 air changes per hour in the animal room. The photoperiod was 12 h light / 12 h dark (with the help of fluorescent lighting), except for necessary dose adjustments and data collection periods. Make routine daily observations. From arrival at the laboratory, approximately 180 g of ...
Embodiment 2
[0274] Example 2. The role of peptides in protecting humans from vascular occlusive injury
[0275] This example will determine the administration of D-Arg-2'6'-Dmt-Lys-Phe-NH at the time of revascularization 2 Does it limit infarct size during acute myocardial infarction.
[0276] research group. Men and women 18 years of age or older who presented to the hospital after the onset of chest pain and had a clinical decision to treat them with revascularization (eg, PCI or thrombolysis) were eligible for inclusion. Patients can be STEMI (ST-segment elevation myocardial infarction) or non-STEMI. A STEMI patient will have symptoms suggestive of a cut off of blood supply to the heart muscle and conditioned by the typical heart attack pattern where the patient's ECG shows ST-segment elevation. Therefore, the diagnosis is based solely on symptoms, clinical examination, and ECG changes. In the case of a non-ST-segment elevation heart attack, the symptoms of chest pain can be the ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap