A testing kit for detecting aquatic animal virus
A technology for aquatic animals and detection reagents, which is applied in the directions of magnetic particle immunodiagnostic reagent carriers, measuring devices, biological material analysis, etc., can solve problems such as hindering the popularization of NNV method, difficulty in large-scale testing, and inability to promote technology.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0038] Example 1-2 Extraction of iridescent virus (GIV) from grouper larvae
[0039] Another specific embodiment of the present invention is the extraction of Irido Virus (GIV) from grouper larvae. Put 0.1 g of the removed pre-kidney tissue into a microcentrifuge tube, and add 200μl of extract (MagQuCo., Ltd.) to the microcentrifuge tube. In order to keep the tissue freshness, place the microcentrifuge tube on ice and place the tissue Grind, 3 minutes after grinding, remove the microcentrifuge tube on ice for 5 minutes, take out the upper solution of the tissue-containing liquid in the microcentrifuge tube, do an immunomagnetic reduction test and measure the GIV concentration.
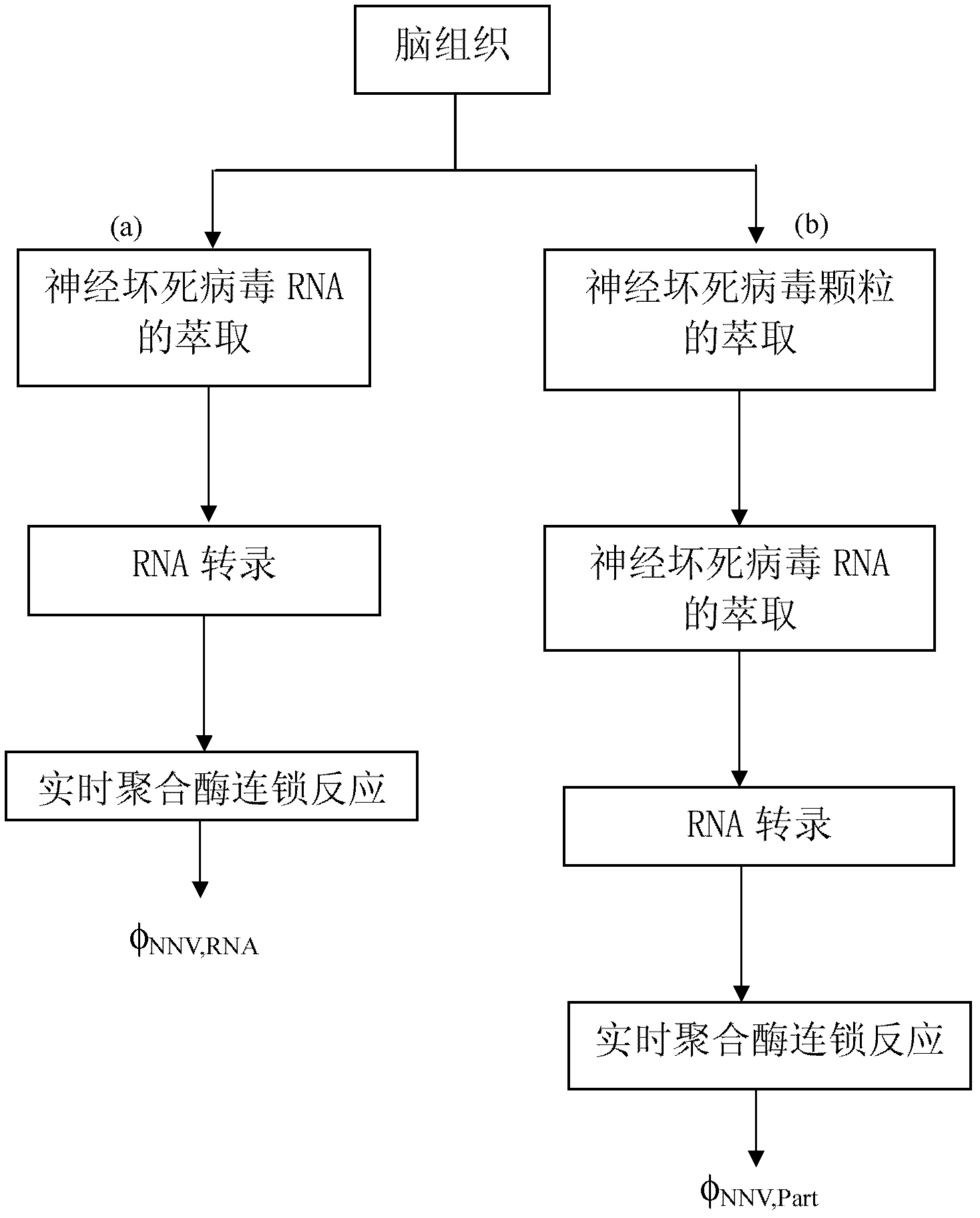
[0040] Figure 1-2 Is a flowchart of the extraction process of different grouper tissues, in which Figure 1-2 (a) The RNA of the iridescent virus is directly extracted from the pre-kidney tissue of the grouper, the extracted RNA is transcribed into DNA, and the DNA concentration is detected by real-time po...
Example Embodiment
[0042] Example 2 Reagent synthesis
[0043] 2-1 Synthesis of magnetic nanoparticles with neuronecrosis virus antibodies
[0044] Mixed stoichiometric ratio of 1:2 ferrous sulfate heptahydrate (FeSO 4 ·7H 2 O) and ferric chloride hexahydrate (FeCl 3 ·6H 2 O) The iron solution is mixed with an equal amount of aqueous dextran, which acts as a surfactant for the ferroferric oxide particles and is dispersed in water. The mixture is heated to 70-90°C, and titrated with a strong base solution to form black ferroferric oxide particles. Aggregates and excess dextran are removed by centrifugation, and the high content is obtained by gel filtration chromatography. Magnetic fluid with homogeneous concentration. The reagent can be diluted with a high-concentration magnetic fluid with a pH of 7.4 phosphate buffer to obtain a suitable magnetic concentration.
[0045] In order to make the antibody bind to the dextran on the outer layer of the magnetic nanoparticles, taking the neuronecrosis virus ...
Example Embodiment
[0055] Example 3 Establishment of Neural Necrosis Virus Concentration and Immune Magnetic Decrement Signal
[0056] In this example, 40 μl and 60 μl of the sample solution were mixed in a glass tube, and a magnetic immunoassay analyzer (XacPro-E, MagQu) was used to measure the unbound neuronecrosis virus-neuronecrosis virus antibody-dextran-nanomagnetic particles. Χ ac, 0 After that, the mixture is maintained at room temperature to form neuronecrosis virus-neuronecrosis virus antibody-dextran-nanomagnetic particles, and the communication signal is measured and recorded χ ac, φ , Χ measured by ac, 0 And χ ac, φ , The following formula (2) can be used to calculate the IMR signal:
[0057] IMR(%)=(χ ac, o -χ ac, φ ) / χ ac, o ×100%....... Formula (2)
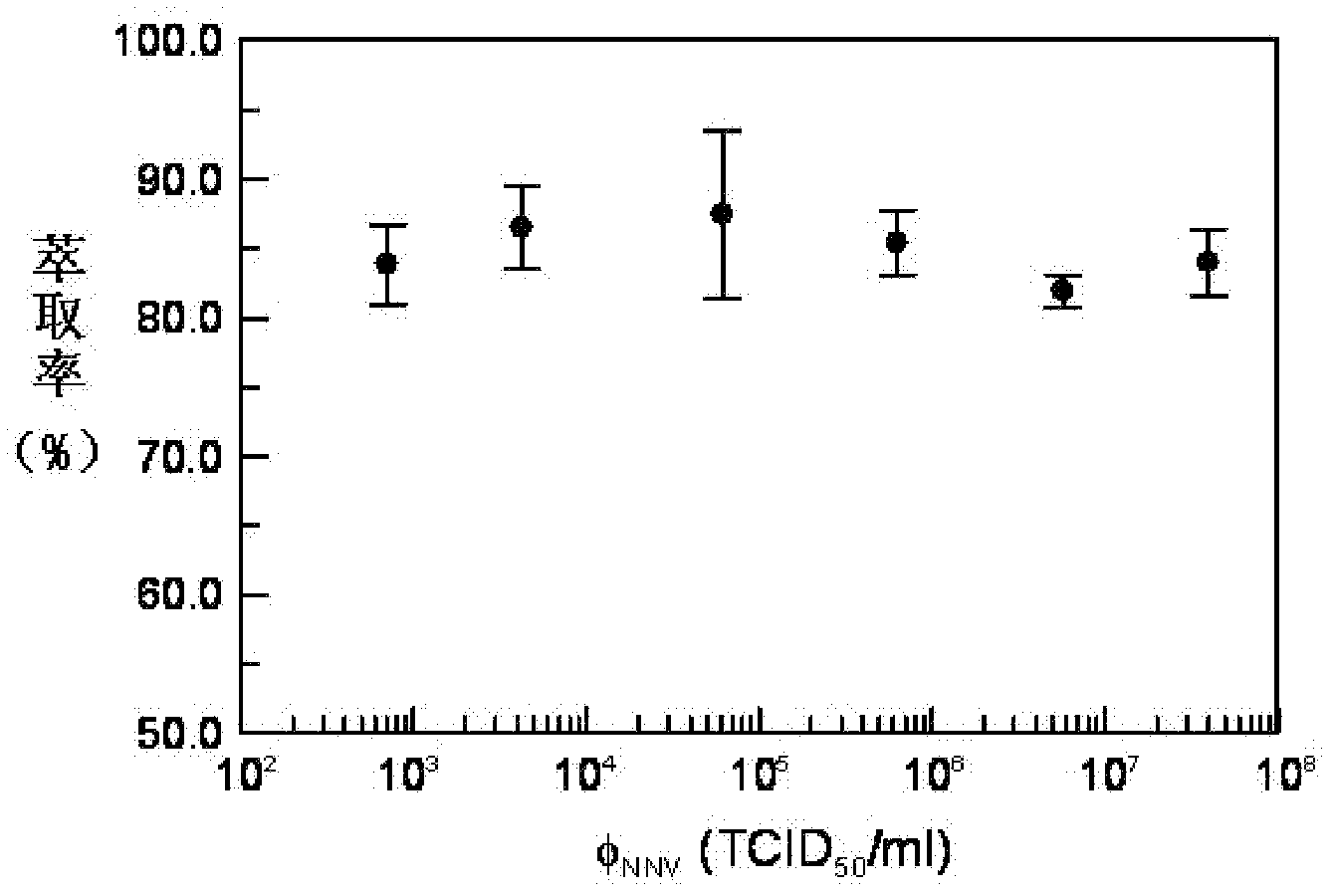
[0058] For each sample solution of a given concentration, the time-dependent χ ac AC signals are all detected for three repetitions. Picture 9 Show the concentration of neuronecrosis virus φ NNV The relationship curve with IMR sign...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap