Optimized monoclonal antibodies against tissue factor pathway inhibitor (tfpi)
A monoclonal antibody, tissue factor technology, applied in the direction of antibodies, anti-peptide structure protease inhibitor immunoglobulin, antibody medical components, etc., can solve the problem of long duration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Example 1: Cloning, Expression and Quantification of Antibody Expression Levels
[0130] The heavy and light chains of wild-type Fabs 2A8 and 4B7 carrying a c-myc tag and a hexahistidine tag at the C-terminus of the heavy chain were subcloned into the pET28a bacterial expression vector (Novagen / Merck Chemicals Ltd., Nottingham, UK), and transformed into Top 10F cells (Invitrogen GmbH, Karlsruhe, Germany). Alternatively, other bacterial expression vectors (eg, pQE vector system, Qiagen GmbH, Hilden, Germany) and strains (eg, DH5α, Invitrogen GmbH, Karlsruhe, Germany) can be used. Variants were generated by standard oligomer-based site-directed mutagenesis and confirmed by DNA sequencing. Specifically, amino acid residues in or around the complementarity determining regions are modified within the heavy and / or light chains.
[0131] To be able to use wild-type or mutant antibodies as competitors, standard PCR-based techniques were used to remove or replace the epitope t...
Embodiment 2
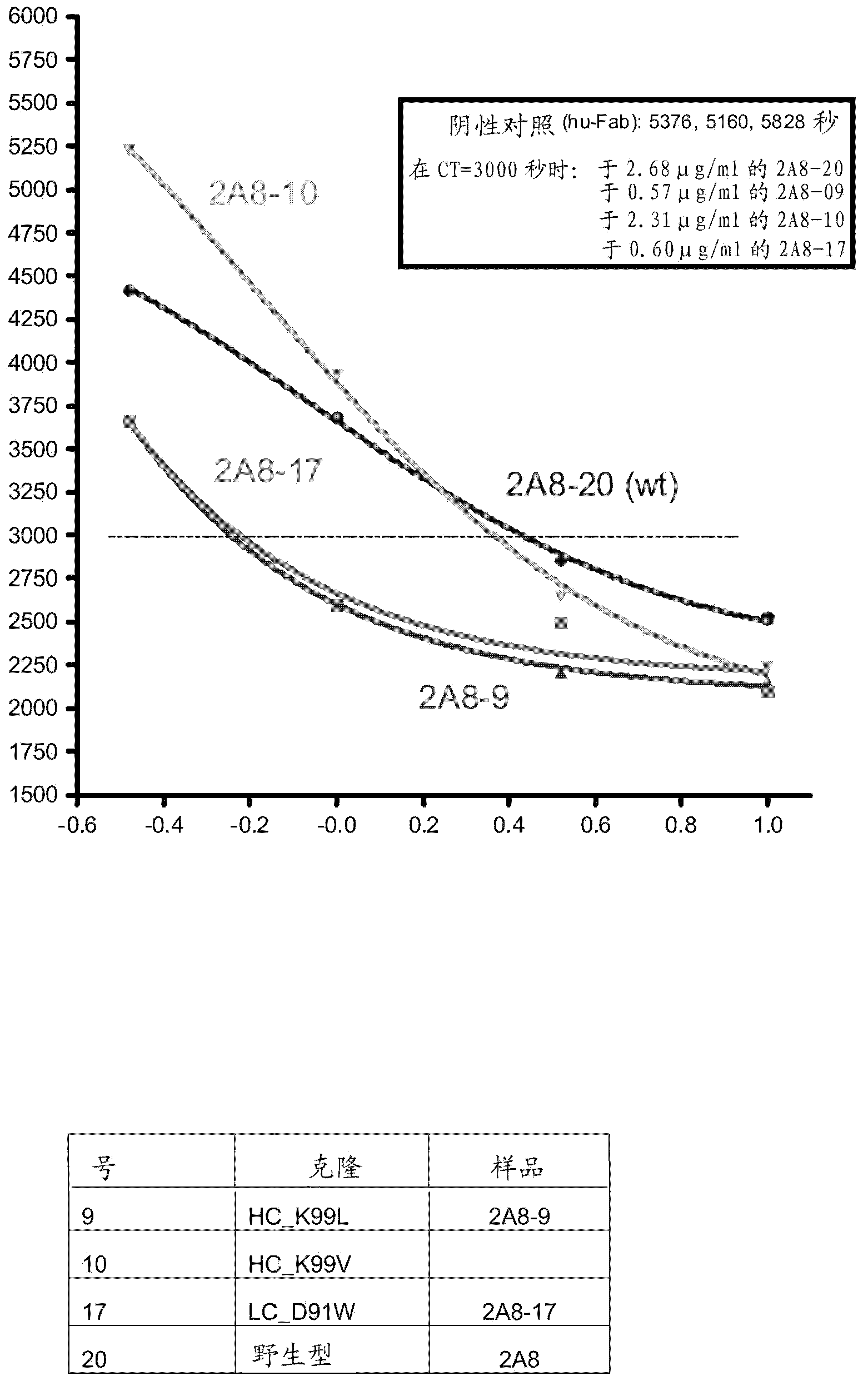
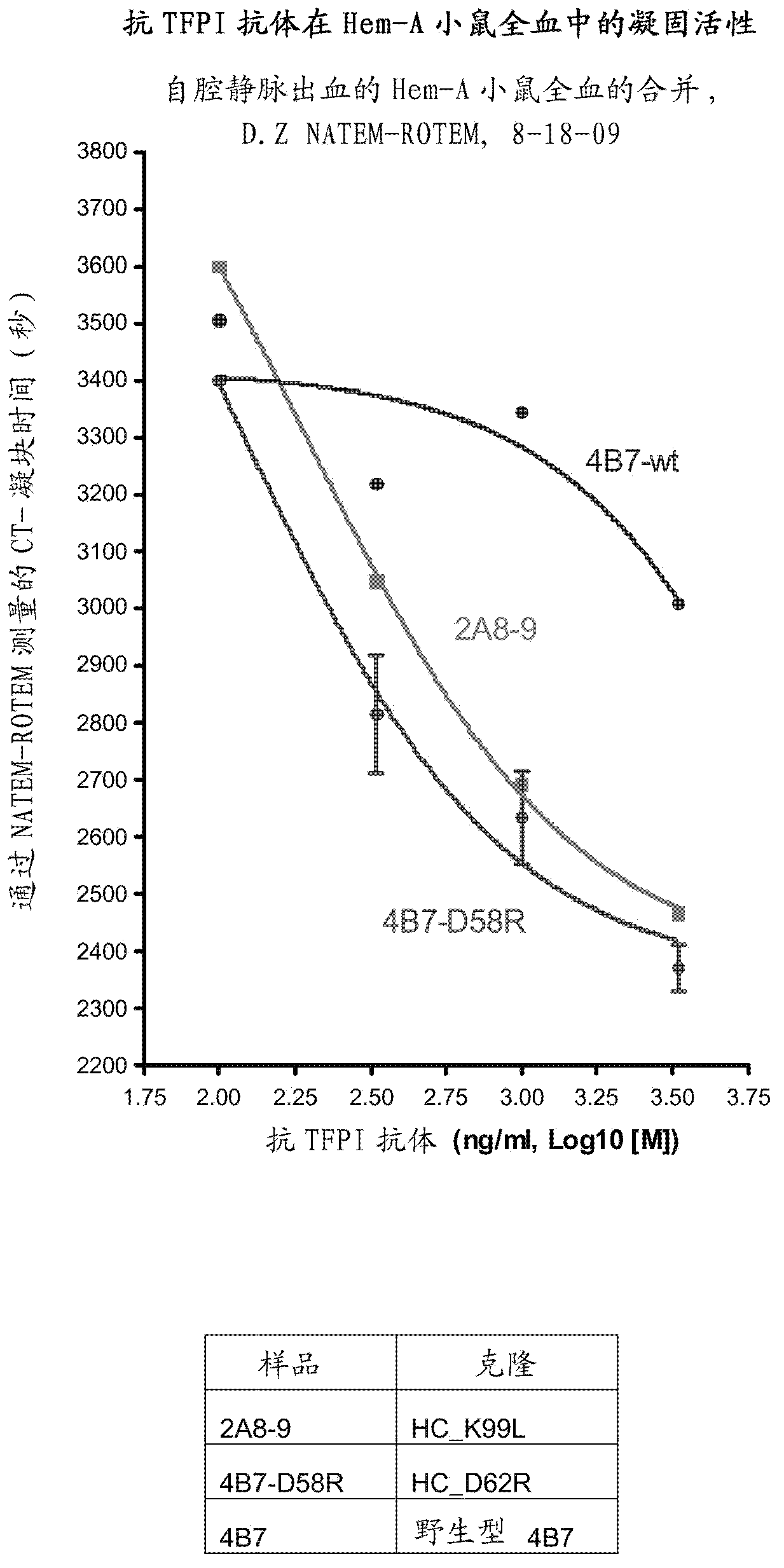
[0134] Example 2: Determination of activity and cross-species cross-reactivity of generated antibody variants
[0135] To determine the activity of mutated antibody variants against human or mouse TFPI (American Diagnostica, 4900B and R&D Systems, 2975-P1, respectively), equilibrium or competitive ELISA assay formats were used. Briefly, MTP plates (Mesoscale Discovery, L21XA-4 or Nunc maxisorp black (black), 460518), and incubated overnight at 4°C. After washing, plates were blocked with 3% bovine serum albumin (Sigma, A4503) in PBST for 1 hour at room temperature and the washing step was repeated. For antibody binding, 10-25 μl of culture supernatant normalized to their corresponding antibody expression levels (if not indicated otherwise) was added to the plate for 1 hour at RT, followed by washing with PBST. Bound wild-type and variants are then detected by epitope tag-specific antibodies or a competition step is included. For competition, 50-300 nM competitor or free ant...
Embodiment 3
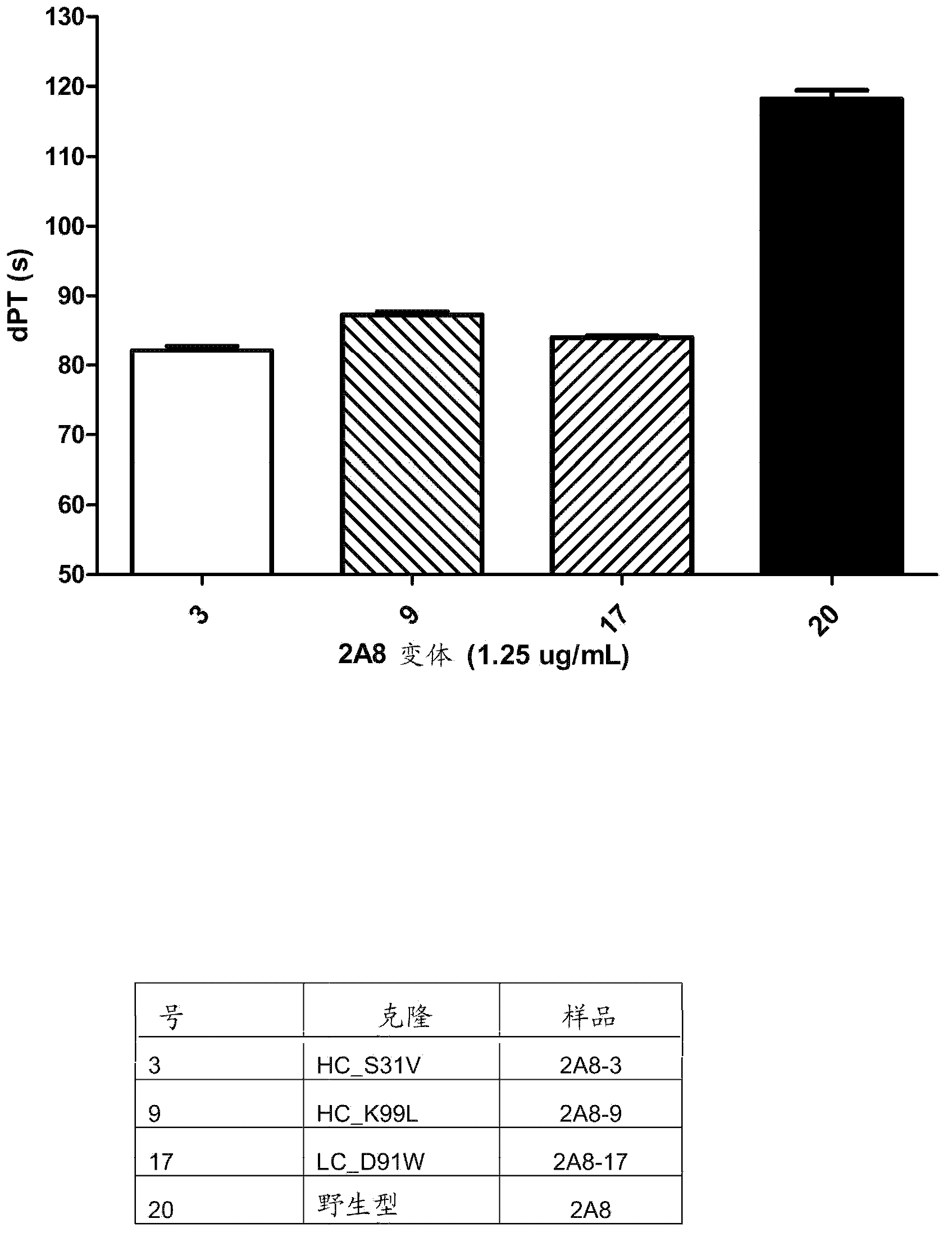
[0136] Example 3: Single and Multiple Amino Acid Substitutions
[0137] Several examples of single amino acid substitutions introduced in the 2A8 heavy or light chain are provided in Table 1. Expression levels of variants were analyzed in quadruplicate in a quantification ELISA. Human and murine TFPI were analyzed for performance in quadruplicate in a competition ELISA after normalization to the corresponding expression levels, and the variant to wild type (wt) ratio was determined. Calculates the error from the standard deviation through error propagation.
[0138] Table 1: Analysis of single amino acid substitutions within 2A8.
[0139]
[0140]
[0141] Some examples of amino acid substitutions combined in the 2A8TFPI antibody are provided in Table 2. Although not every combination is provided in Table 2, it is contemplated that the TFPI antibody may comprise any combination of the modifications provided. Expression levels of variants were analyzed in quadruplicat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com