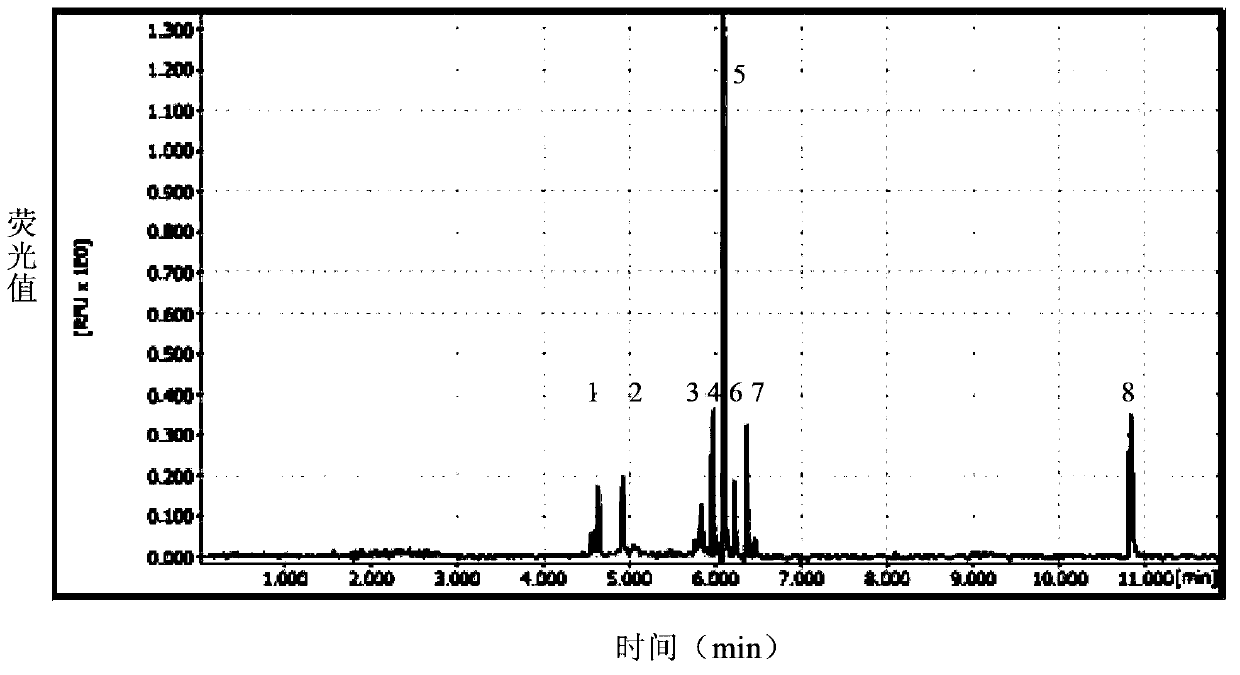
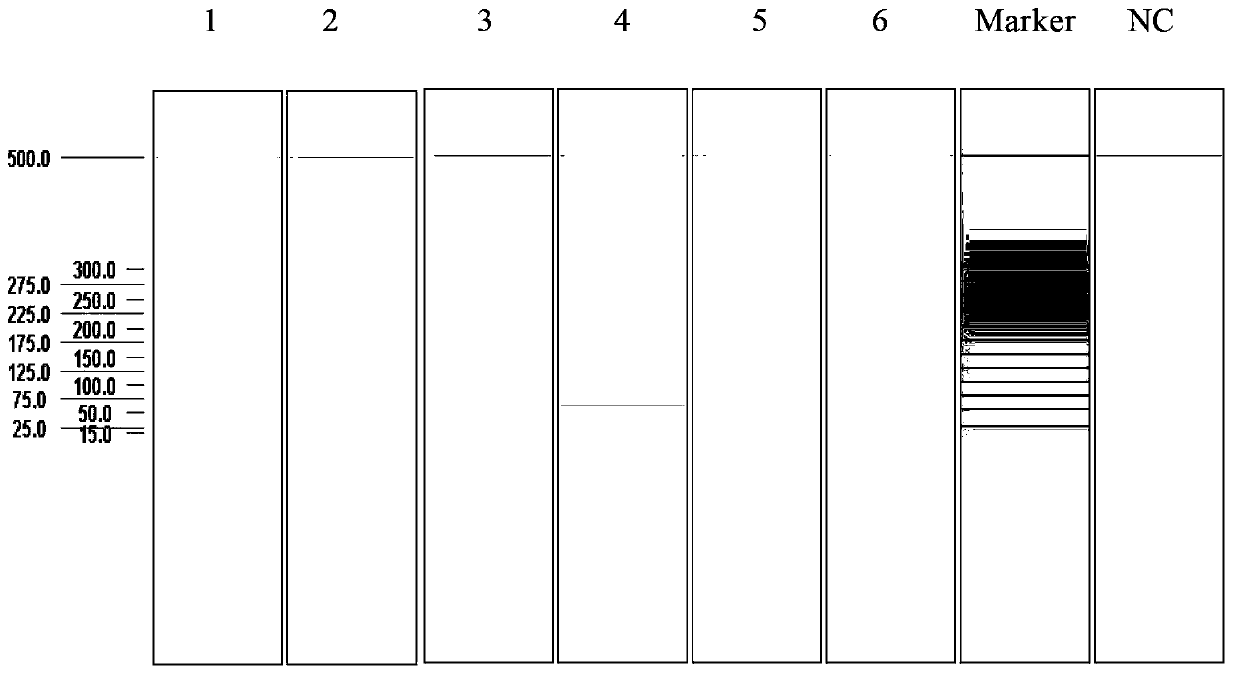
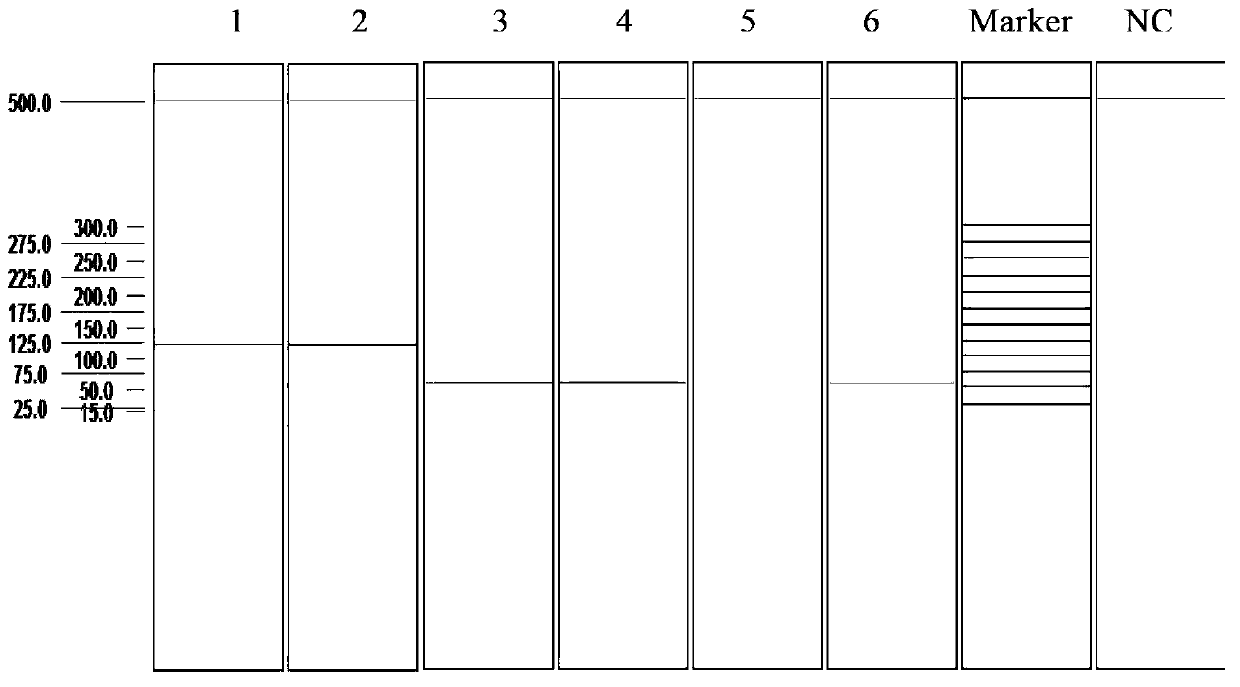
Multiplex ligation-dependent probe amplification detection kit for simultaneously detecting five swine disease viruses, primers and probes
A swine flu virus and virus technology, applied in the field of inspection and quarantine, can solve the problems of long time, difficult to judge the results, heavy detection workload and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1. Preparation and use of kit
[0076] 1. The composition of the kit is shown in Table 4.
[0077] Table 4 The composition of the kit
[0078] Composition (30Tests / box)
Quantity
MLPA buffer
600μL×1 tube
Probe mix
600μL×1 tube
Connection reaction solution
1240μL×1 tube
Ligase-65 ligase (5U / μL)
115μL×1 tube
PCR reaction solution (including universal primers)
750μL×1 tube
SALSA polymerase (5U / μL)
65μL×1 tube
DEPC water
1mL×3 tubes
Negative control
1mL×3 tubes
Positive control
1mL×3 tubes
[0079] Among them, the MLPA buffer was purchased from The MRC-Holland Company, which includes KCl, Tris-HCl, EDTA and PEG-6000, and has a pH of 8.5.
[0080] Probe mixture, which includes long and short probes of swine influenza virus, porcine reproductive and respiratory syndrome virus, pseudorabies virus, transmissible gastroenteritis virus and foot-and-mouth disease virus. The sequence of each probe is shown in Table 1. The 5'ends of the SEQ ID NO: 2, ...
Embodiment 2
[0111] Example 2. Sensitivity test of the kit
[0112] 1. Material:
[0113] Swine influenza virus A / swine / 2003 (H1N1), porcine reproductive and respiratory syndrome JXA1 strain inactivated virus, pseudorabies virus Nanyang strain, porcine transmissible gastroenteritis virus purdue115 international standard strains are preserved in the laboratory, O type foot and mouth disease is inactivated Live virus was provided by Lanzhou Veterinary Research Institute of Chinese Academy of Agricultural Sciences.
[0114] 2. Method
[0115] 1) Preparation of in vitro transcribed RNA and plasmid DNA
[0116] Preparation of swine influenza virus M gene in vitro transcription RNA: recovery of the RT-PCR amplification product of swine influenza virus A / swine / 2003 (H1N1) strain M gene, length of 982bp, and pGEM-T vector (purchased from PromeGA) Ligation, transformation of JM109 competent cells, alkaline lysis method to extract plasmid DNA, PCR and restriction enzyme digestion to obtain a positive recomb...
Embodiment 3
[0132] Example 3. Specificity test of the kit
[0133] 1. Material
[0134] Table 6 Viruses and nucleic acids used in the study of specificity tests
[0135] Virus
Source
Swine flu virus A / Swine / 2003(H1N1)
Saved in this laboratory
Porcine reproductive and respiratory syndrome JXA1 strain inactivated virus
Saved in this laboratory
Nanyang strain of pseudorabies virus
Saved in this laboratory
Swine transmissible gastroenteritis purdue115 international standard strain
Saved in this laboratory
O-type foot-and-mouth disease inactivated virus
Lanzhou Veterinary Research Institute
Porcine parvovirus
Saved in this laboratory
Swine fever inactivated virus
Provided by China Veterinary Drug Supervision Institute
Porcine epidemic diarrhea virus
Saved in this laboratory
[0136] 2. Method
[0137] 2.1 Use any set of probes among the five viruses of swine influenza virus, porcine reproductive and respiratory syndrome virus, pseudorabies virus, porcine transmissible gast...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com