Compound diphenoxylate tablet and preparation method thereof
A technology for phenoxylate tablets and diphenoxylate hydrochloride is applied in the field of compound diphenoxylate tablets and their preparation, which can solve the problems of unsatisfactory content uniformity, low dissolution rate of compound diphenoxylate tablets, etc. Effects of dissolution and content uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
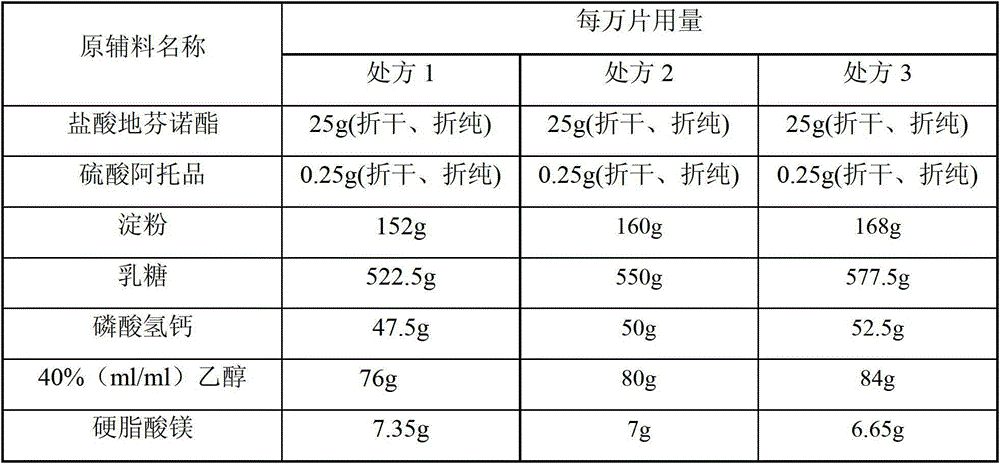
[0018] Embodiment 1 Compound Diphenoxylate Tablet Prescription
[0019] Table 1
[0020]
Embodiment 2
[0021] The preparation of embodiment 2 compound diphenoxylate tablets
[0022] 1. Crushing and sieving of raw and auxiliary materials: The crushing of raw and auxiliary materials is completed with the help of a universal high-speed pulverizer; the sieving is done with a vibrating sieve. The raw material diphenoxylate hydrochloride and auxiliary materials such as starch, magnesium stearate, and calcium hydrogen phosphate are required to be sieved through an 80-mesh sieve with a vortex oscillator; the lactose auxiliary material is required to be crushed with a universal high-speed pulverizer and then sieved through an 80-mesh sieve with a vortex oscillator;
[0023] 2. Weighing: first weigh the auxiliary material starch with the same weight as diphenoxylate hydrochloride and put it into the container for mixing and dilution, pass through a 80 mesh sieve, then weigh the starch with the same weight as the mixture, and pass through 80 mesh Sieve to obtain a well-mixed mixture;
[...
Embodiment 3
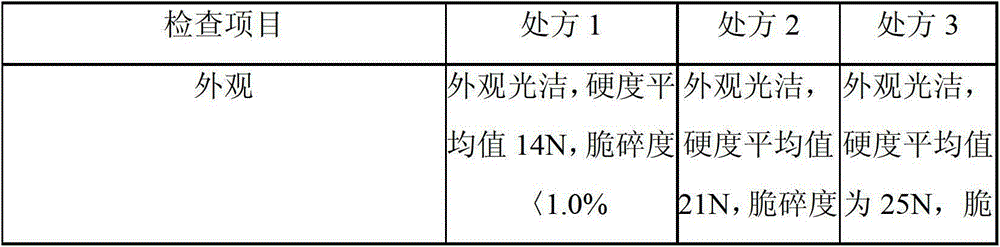
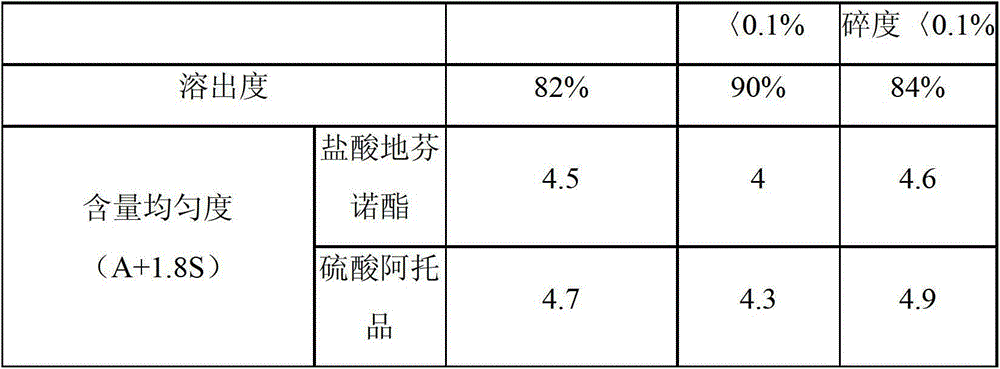
[0032] Embodiment 3 according to the item inspection of the compound diphenoxylate sheet prepared by the prescription of embodiment 1 and embodiment 2 method
[0033] Dissolution: Dissolution medium: acetic acid aqueous solution containing 0.2mol / L; using device 1, rotating speed 150rpm, time 45 minutes, detection method: mobile phase: acetonitrile: potassium dihydrogen phosphate (65:35), standard solution: the prepared concentration is Diphenoxylate hydrochloride standard solution of 250ug / ml, dilute to 500ml with dissolution medium, filter, chromatographic system; 210nm wavelength detector, L11 packed column (3.9mm*30mm), flow rate: 1.0ml / min, standard Inject the solution, record the chromatogram, the tailing factor shall not exceed 1.5, and the relative standard deviation shall not exceed 2.0%, inject the standard solution and the test product separately, the injection volume is 50ul, record the chromatogram, and the dissolution rate shall not be low within 45 minutes 75% o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| friability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com