Crystal structure of neuropilin fragment in complex with neuropilin antibody
A complex and fragment technology, applied in the field of crystal structure of neuropilin fragment and neuropilin antibody complex, can solve problems such as expression inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0177] Based on the domain architecture and functional characteristics, the extracellular domain of Nrp is usually divided into three functional units: a1a2, b1b2, and c, which represent their main brain signal protein binding, VEGF binding, and dimerization regions ( Ellis, LM, Mol Cencer Ther 5, 1099-107 (2006)). Although structure-function studies describe the overall domain requirements of neuropilin function, the molecular details of these receptor / ligand complexes are still poorly understood. So far, structural information about Nrp is limited to the b1b2 domain of Nrp1 (Vander Kooi et al., supra; Lee, C. et al., J Biol Chem 281, 5702-10 (2006)). The crystal structure of Nrp1 b1b2 complexed with Tuftsin (a tetrapeptide homologous to the C-terminus of VEGF165) provides first-hand structural information about the interaction of Nrp with its binding partner VEGF (VanderKooi, et al., see above ; Von Wronski, MA, et al., J. Biol Chem 281, 5702-10 (2006)).
[0178] The present ...
Embodiment 1
[0309] Example 1: Anti-Nrp1 B And anti-pan Nrp A Construction and function of antibodies
[0310] Liang et al., J Mol Biol 366, 815-29 (2007) and Pan et al., Cancer Cell 11, 53-67 (2007) reported a strategy for developing phage-derived antibodies that selectively block brain signaling protein or VEGF binding to Nrp1. These monoclonal antibodies are designed as tools for distinguishing Nrp1-mediated various responses and for evaluating their potential as therapeutic agents in murine tumor models.
[0311] In short, a human synthetic antibody phage library was designed, which was built with VH / VL diversity on a single consensus scaffold. The details of the design and selection of this antibody library are described in Liang et al., see above, and can also be found in WO 03 / 102157 published on December 11, 2003, the complete disclosure of which is incorporated herein by the above. Since then, the library has generated functional blocking antibodies against human and murine NRP1. The...
Embodiment 2
[0314] Example 2: Structure study of neuropilin / antibody complex
[0315] Materials and Method
[0316] Functional assays and antibody binding affinity
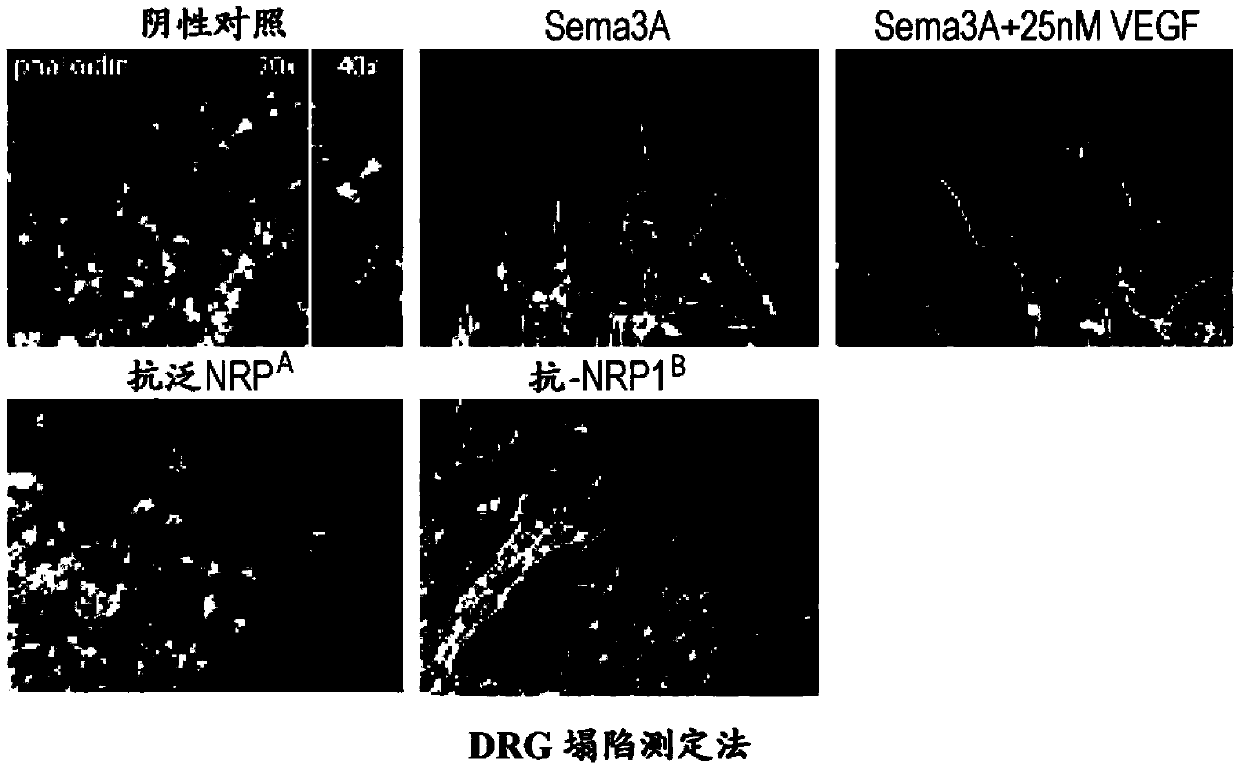
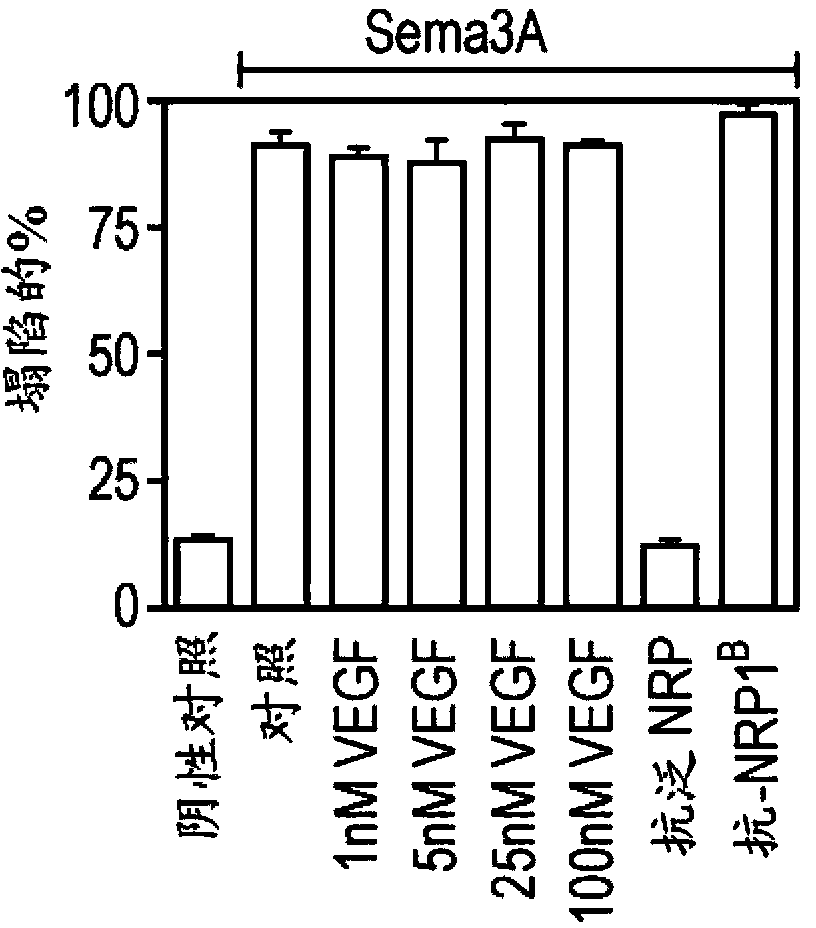
[0317] The collapse assay and the determination of anti-Nrp antibody binding affinity were performed as previously described (He, Z and Tessier-Lavigne, M., Cell90, 739-51 (1997); Liang et al., supra and Pan et al., supra Text).
[0318] Expression and purification of proteins for crystallographic research
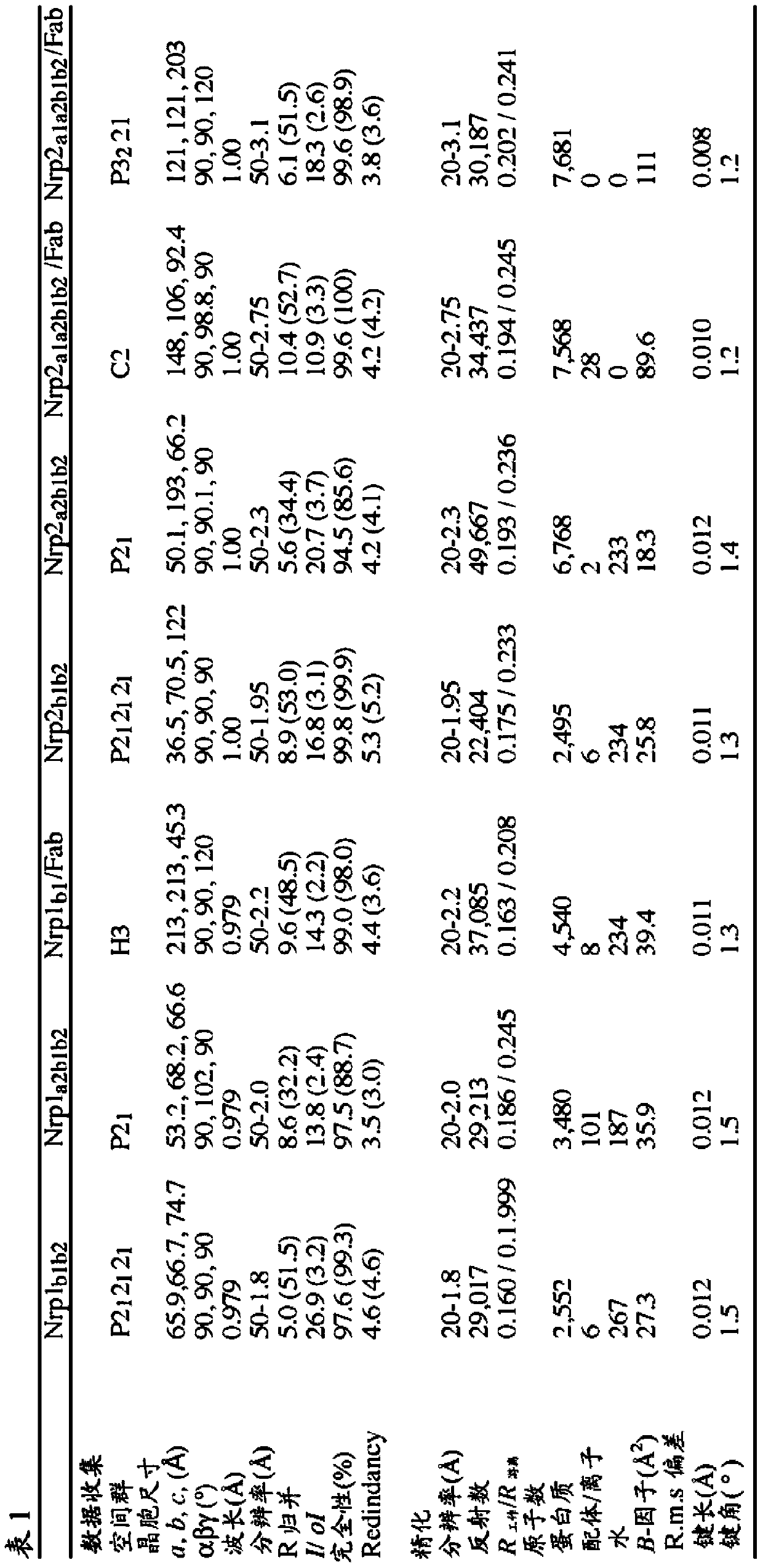
[0319] Nrp1-b1, Nrp1-b1b2, and Nrp2-b1b2 (see Table S1 for domain boundaries) were cloned into pET15b (Novagen) and expressed in E. coli at 37°C (Nrp1-b1 and -b1b2) or 16°C (Nrp2-b1b2) Incubate. All neuropilin b-type fragments are expressed as soluble proteins and do not require a refolding scheme. After the cells were lysed, the protein was purified using tweezers-nitrilotriacetic acid (Ni-NTA) resin in 50 mM Tris (pH 8.0), 300-500 mM NaCl, and 20 mM imidazole, and eluted in the same buffer plus 250 mM imidazole. Use thrombin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com