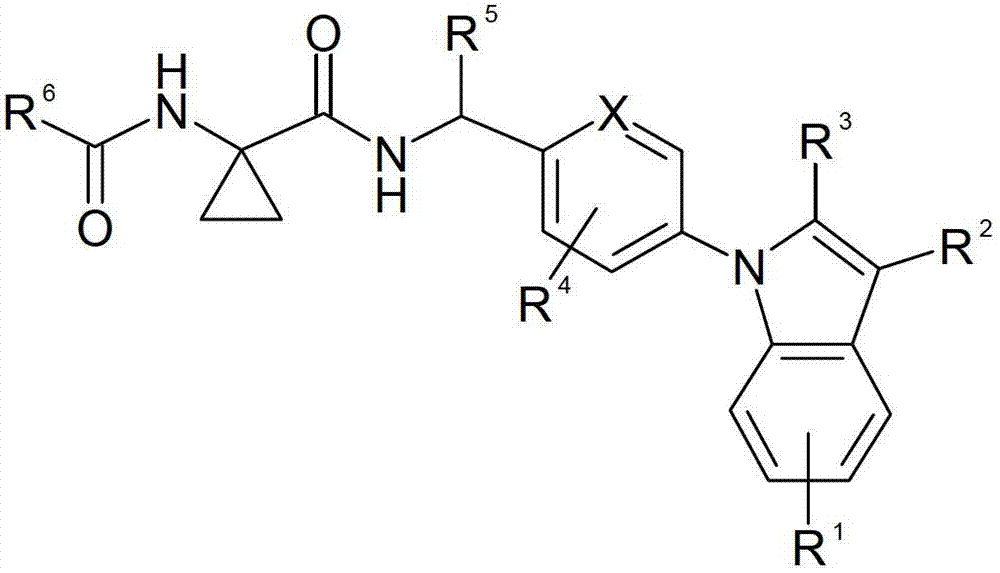
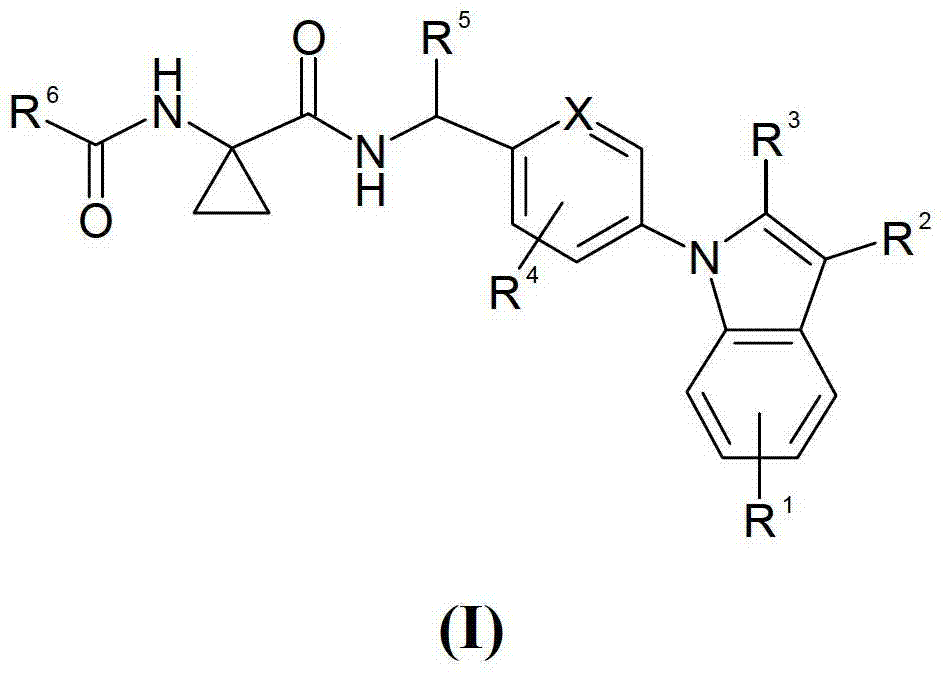
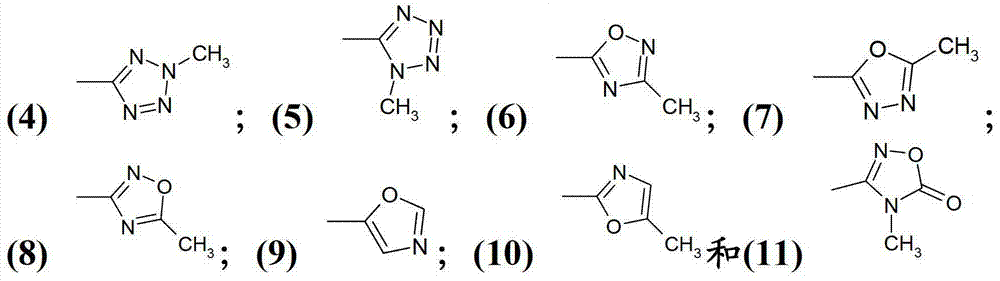
Indole derivatives
A technology of indole derivatives and indole, which is applied in the field of indole derivatives and can solve problems such as undisclosed compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0146] The foregoing ingredients and various preparation routes are representative only. Other materials and process techniques known in the art can also be used.
[0147] The compounds of the present invention are bradykinin receptor antagonists, in particular selective bradykinin B1 receptor antagonists, and are therefore useful in the treatment or prevention of conditions including pain and inflammation, which include feeding into the breast to be treated An effective amount of the compound of formula (I) of the present invention is administered to the animal as it is or in the form of a medicament.
[0148] The compounds will be effective in the treatment of musculoskeletal pain and disorders of the body, including bone and joint pain and disorders (e.g., arthritis, including rheumatoid and other types of inflammatory arthritis, osteoarthritis , spondylitis and infectious arthritis, arthritis caused by gout or pseudogout, autoimmune and vasculitic joint disorders such as ...
Embodiment 1
[0469] 1-(2,2,2-Trifluoro-acetylamino)-cyclopropanecarboxylic acid 4-[3-chloro-2-(2-methyl-2H- Tetrazol-5-yl)-indol-1-yl]-benzylamide
[0470] To 4.0g (8.7mmol) 1-amino-cyclopropanecarboxylic acid 4-[3-chloro-2-(2-methyl-2H-tetrazol-5-yl)-indol-1-yl]-benzyl In a stirred solution of amide hydrochloride (reference example 4) and 3.6mL (25.8mmol) triethylamine in 180mL dichloromethane, 1.36mL (9.8mmol) trifluoroacetic anhydride was added dropwise below 20°C, and the reaction The mixture was stirred at room temperature for 3 h. Then 100 mL of water was added to the mixture, the organic layer was separated, dried over anhydrous sodium sulfate and concentrated in vacuo. The residue was subjected to flash column chromatography using silica gel 60 (0.015-0.040 mm) as adsorbent (Merck) and toluene:acetone = 2:1 as eluent to give after crystallization from 2-propanol 2.2 g (48.7% ) of the title compound. MS(EI)518.2(MH + ).
Embodiment 2
[0472] 3-methoxy-iso Azole-5-carboxylic acid (1-{4-[3-chloro-2-(2-methyl-2H-tetrazol-5-yl)- Indol-1-yl]-benzylcarbamoyl}-cyclopropyl)-amide
[0473] 0.16g (0.42mmol) 4-[3-chloro-2-(2-methyl-2H-tetrazol-5-yl)-indol-1-yl]-benzylamine hydrochloride (reference example 4) , 0.107g (0.47mmol) 1-[3-methoxy-iso Azole-5-carbonyl)-amino]-cyclopropanecarboxylic acid (P.D.O'Shea et al. J.Org.Chem. 2009, 74, 4547-4553), 0.15mL (1.07mmol) triethylamine, 0.192g (0.5 A mixture of mmol) HBTU and 5 mL of N,N-dimethylformamide was stirred at room temperature for 24 h, then concentrated in vacuo. The residue was subjected to flash column chromatography using silica gel 60 (0.015-0.040 mm) as adsorbent (Merck) and toluene:methanol = 4:1 as eluent to give 0.12 g (51.5%) of the title after crystallization from diethyl ether compound. MS(EI)548.1(MH + ).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap