One-pot method for synthesizing chiral amino boronate intermediate
A technology for amino borate and intermediates, which is applied in the field of one-pot synthesis of chiral amino borate intermediates, which can solve problems such as difficult long-term storage, increased costs, and capacity limitations, and achieve the effect of simple operation and increased costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
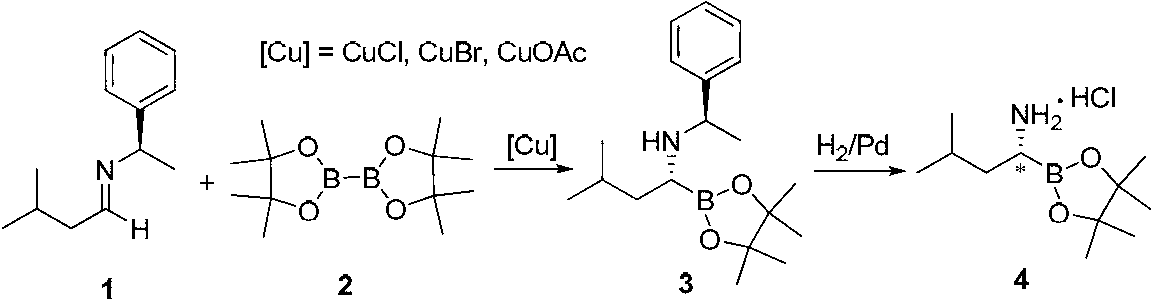
[0040] Add 1.21g (10mmol) of (R)-tert-butylsulfinamide, 1.03g (12mmol) of isovaleraldehyde and 2.71g (12mmol) of neopentyl glycol diborate into a 100ml reaction bottle, then add 50ml of tert-butyl methyl ether and stir Disperse and mix well, add 2g of anhydrous magnesium sulfate and 329mg (1mmol) of copper trifluorosulfonate under the atmosphere of nitrogen protection. After the addition is complete, let the reaction mixture react at room temperature for 48 hours. TLC monitors the progress of the reaction. After the reaction is completed, , add 50ml ethyl acetate to disperse the reaction liquid, then add 1N NaHCO 3 3 liters of aqueous solution was washed once, the upper organic phase was washed three times with saturated brine, the aqueous phase was separated and discarded, the organic phase was stirred and dried with an appropriate amount of anhydrous sodium sulfate, and the organic phase was concentrated under reduced pressure to obtain the compound R-N-(R-tert-bu...
Embodiment 2
[0042]
[0043] Add 1.21g (10mmol) of (S)-tert-butylsulfinamide, 1.03g (12mmol) of isovaleraldehyde and 2.71g (12mmol) of neopentyl glycol diborate into a 100ml reaction bottle, then add 50ml of dichloromethane and stir Disperse and mix well, add 2g of anhydrous sodium sulfate and 180mg (1mmol) of copper propionate under the atmosphere of nitrogen protection, after the addition is complete, let the reaction mixture react at 0°C for 48 hours, monitor the progress of the reaction by TLC, after the completion of the reaction , add 50ml ethyl acetate to disperse the reaction liquid, then add 1NNaHCO 3 3 liters of aqueous solution was washed once, the upper organic phase was washed three times with saturated brine, the aqueous phase was separated and discarded, the organic phase was stirred and dried with an appropriate amount of anhydrous sodium sulfate, and the organic phase was concentrated under reduced pressure to obtain the compound S-N-(S-tert-butyl Sulfonyl)-1-amino-3-me...
Embodiment 3
[0045]
[0046] Add 1.21g (10mmol) of (R)-tert-butylsulfinamide, 1.03g (12mmol) of isovaleraldehyde and 3.04g of (2-methyl-2,4-pentanediol) diboronic acid into a 100ml reaction bottle ( 12mmol), then add 50ml tetrahydrofuran and stir to disperse and mix evenly. Under the atmosphere of nitrogen protection, add 2g of cesium carbonate and 100mg (1mmol) of cuprous chloride. After the addition is complete, let the reaction mixture react at room temperature for 48 hours. , after the reaction is complete, add 50ml of ethyl acetate to disperse the reaction solution, then add 1N NaHCO 3 3 liters of aqueous solution was washed once, the upper organic phase was washed three times with saturated brine, the aqueous phase was separated and discarded, the organic phase was stirred and dried with an appropriate amount of anhydrous sodium sulfate, and the organic phase was concentrated under reduced pressure to obtain the compound R-N-(R-tert-butyl Sulfonyl)-1-amino-3-methylbutane-1-boronic...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap