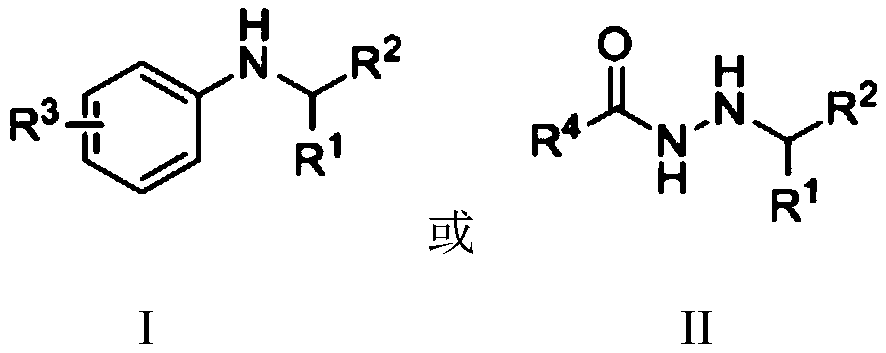
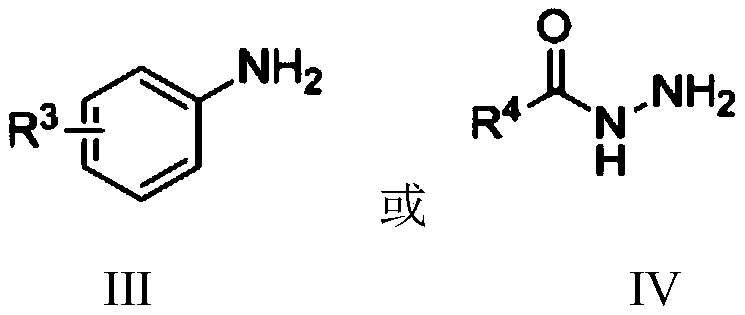
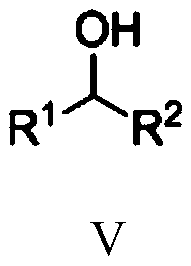
Nickel-catalyzed n-alkylation to prepare secondary amines or n′-alkylhydrazides
A technology of alkylation reaction and alkyl hydrazide, which is applied in the field of N-alkylation, can solve problems such as N'-alkylation of hydrazide compounds, save experimental steps and costs, and improve environmental friendliness performance, easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: Preparation of N-phenyl-1-phenyl-1-ethylamine
[0049]
[0050] Under nitrogen protection, add Ni(OTf) into a 10mL Schlenk reaction tube 2 (2.9mg, 0.008mmol), 1,2-bis(dicyclohexylphosphine)ethane (4.3mg, 0.010mmol) and tert-amyl alcohol (1.2mL), stirred for 10 minutes, then added aniline (38mg, 0.4mmol) , 1-phenylethanol (98mg, 0.8mmol) and Molecular sieves (100 mg). The reaction tube was sealed and heated in an oil bath at 120°C for 24 hours. After the reaction stopped, it was cooled, and the reaction solution was directly added to a silica gel column, and eluted with petroleum ether and ethyl acetate (volume ratio 20:1) to obtain pure N-phenyl-1-phenyl-1-ethylamine, the yield 80%.
[0051] 1 H NMR (400MHz, CDCl 3 ):δ7.41-7.33(m,2H),7.30-7.26(m,2H),7.24-7.18(m,1H),7.12-7.00(m,2H),6.63(m,1H),6.55-6.42 (m,2H),4.47(q,J=6.7Hz,1H),4.00(br s,1H),1.50(d,J=6.7Hz,3H).
[0052] 13 C NMR (101MHz, CDCl 3 ): δ147.3, 145.2, 129.1, 128.6, 126.8, 125.8, 117...
Embodiment 2
[0053] Embodiment 2: Preparation of N-(4-methylphenyl)-1-phenyl-1-ethylamine
[0054]
[0055] Under nitrogen protection, add Ni(OTf) into a 10mL Schlenk reaction tube 2 (2.9mg, 0.008mmol), 1,2-bis(dicyclohexylphosphine)ethane (4.3mg, 0.010mmol) and tert-amyl alcohol (1.2mL), stirred for 10 minutes, then added 4-methylaniline (43mg , 0.4mmol), 1-phenylethanol (98mg, 0.8mmol) and Molecular sieves (100 mg). The reaction tube was sealed and heated in an oil bath at 120°C for 24 hours. After the reaction stopped, it was cooled, and the reaction solution was directly added to a silica gel column, and eluted with petroleum ether and ethyl acetate (volume ratio 20:1) to obtain pure N-(4-methylphenyl)-1-phenyl-1 -Ethylamine, 90% yield.
[0056] 1 H NMR (400MHz, CDCl 3 ): δ7.36(d, J=7.2Hz, 2H), 7.30(t, J=7.1Hz, 2H), 7.23-7.21(m, 1H), 6.89(d, J=7.6Hz, 2H), 6.43 (d, J=7.7Hz, 2H), 4.45(q, J=6.3Hz, 1H), 3.90(s, 1H), 2.18(s, 1H), 1.49(d, J=6.3Hz, 3H).
[0057] 13 C NMR (101MHz,...
Embodiment 3
[0058] Example 3: Preparation of N-(3,5-dimethylphenyl)-1-phenyl-1-ethylamine
[0059]
[0060] Under nitrogen protection, add Ni(OTf) into a 10mL Schlenk reaction tube 2 (2.9mg, 0.008mmol), 1,2-bis(dicyclohexylphosphine)ethane (4.3mg, 0.010mmol) and tert-amyl alcohol (1.2mL), stirred for 10 minutes, then added 3,5-dimethyl Aniline (49mg, 0.4mmol), 1-phenylethanol (98mg, 0.8mmol) and Molecular sieves (100 mg). The reaction tube was sealed and heated in an oil bath at 150°C for 24 hours. After the reaction stopped, cooled, the reaction solution was directly added to the silica gel column, and eluted with petroleum ether and ethyl acetate (volume ratio 20:1) to obtain pure N-(3,5-dimethylphenyl)-1-benzene Base-1-ethylamine, yield 72%.
[0061] 1 H NMR (400MHz, CDCl 3 ): δ7.36(d, J=7.2Hz, 2H), 7.31(t, J=7.2Hz, 2H), 7.25-7.20(m, 1H), 6.31(s, 1H), 6.16(s, 2H) ,4.47(q,J=6.3Hz,1H),3.90(s,1H),2.16(s,6H),1.49(d,J=6.4Hz,3H).
[0062] 13 C NMR (101MHz, CDCl 3 ): δ147.5, 145...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap