Pharmaceutical and food composition for preventing or treating diabetes or obesity
一种糖尿病、肥胖症的技术,应用在药物组合、食品制备、食品成分等方向,能够解决降低选择性和生物学利用可能性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0226] Preparation of Lobaric acid from Lichens Stereocaulon alpinum Extract
[0227] 1-1: Preparation of lichen-like alpine coral branch lichen (Stereocaulon alpinum) extract
[0228] Lichens Stereocaulon alpinum (Hedw.) G.L.Sm.) were collected from Barton Peninsula around King Sejong Station (S62°13.3', W58°47.0') on King George Island, Antarctica in January 2003, It is a lichen that is easily collected in the Barton Peninsula.
[0229] 50 g of dried alpine coral branch lichen (Stereocaulon alpinum) was extracted twice with 1 L of methanol over 24 hours to obtain 3.6 g of methanol extract. The extract obtained above was filled with silica gel (C 18 ), and add 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90% and 100% (v / v) methanol (MeOH), the fractions were obtained separately.
[0230] 1-2: Preparation of Robbalic Acid from Lichen Stereocaulon alpinum Extract
[0231] The 204.6 mg fraction obtained by eluting with 80% methanol in Example 1-1 was repacked into a silica gel (...
Embodiment 2
[0238] Synthetic sodium lobarate
[0239] 2-1: Preparation of Robabaic Acid Sodium from Robabaic Acid
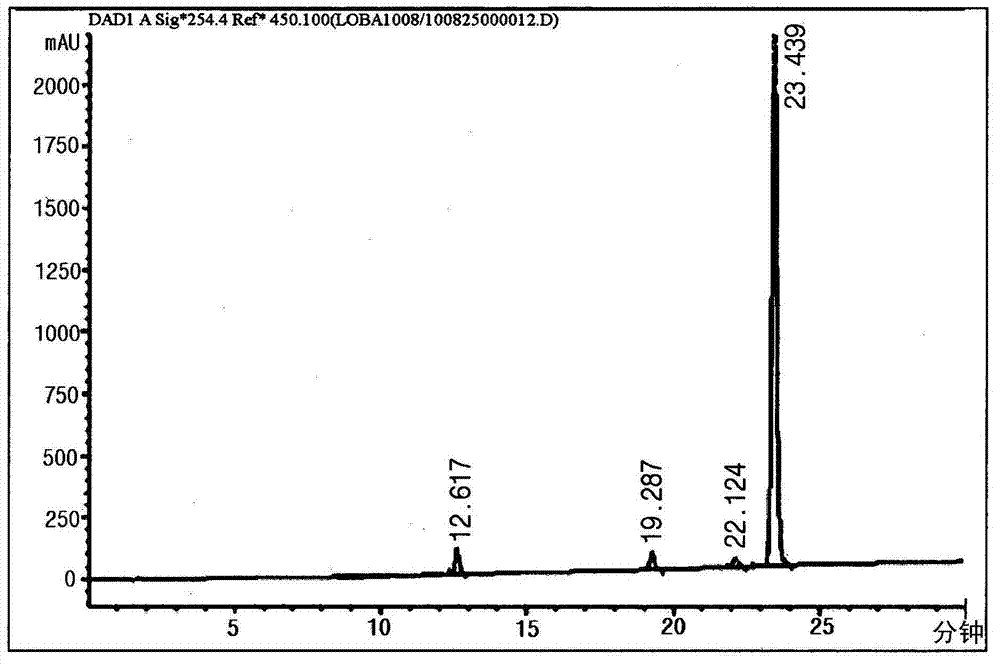
[0240] 10mg (22umol) of Robabaic acid obtained from Example 1-2 was dissolved in 3ml of acetone (Acetone), to which 50ul of 1M NaHCO was added 3 Stir for 1-2 minutes afterwards. For adding NaHCO 3The co-formed solid was filtered and immediately concentrated completely on a rotary evaporator. At the end of the concentration, sodium robalate (figure 1 ). The obtained sodium robalate is 100% dissolved in water, ensuring water solubility,
[0241] chemical formula 2
[0242]
[0243] Molecular formula: C 25 h 27 NaO 8
[0244] Molecular weight: 478.47
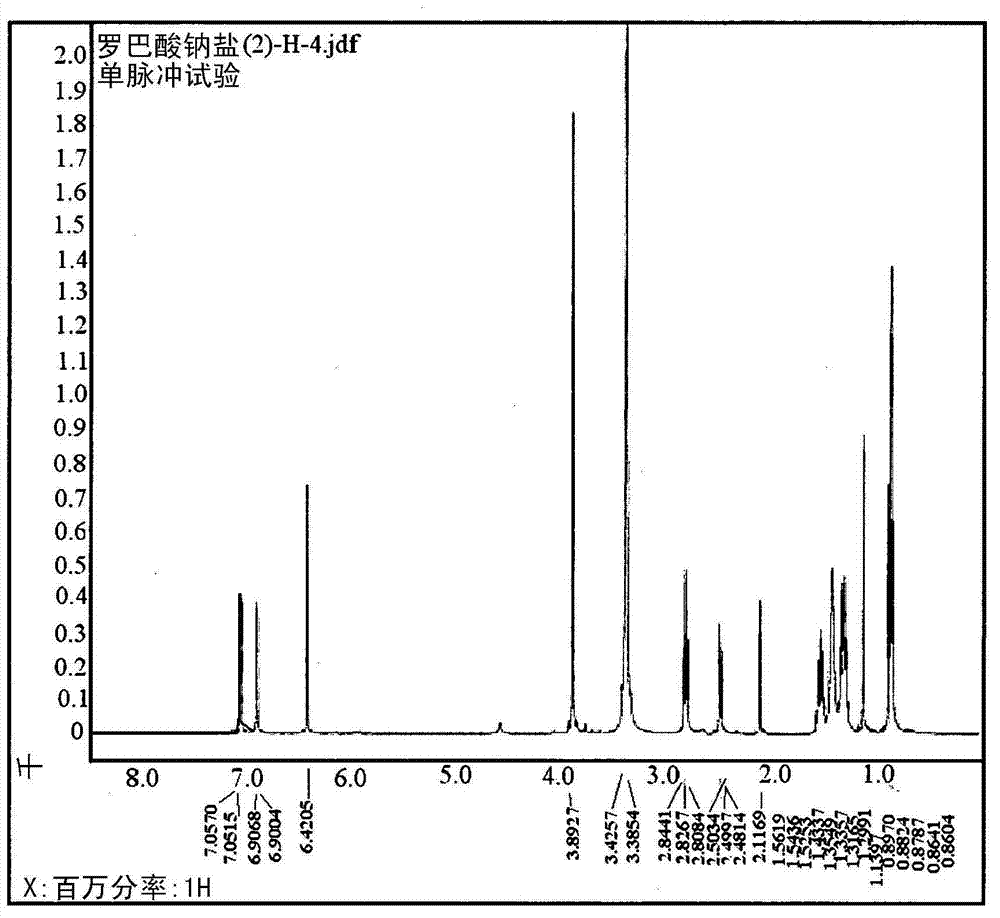
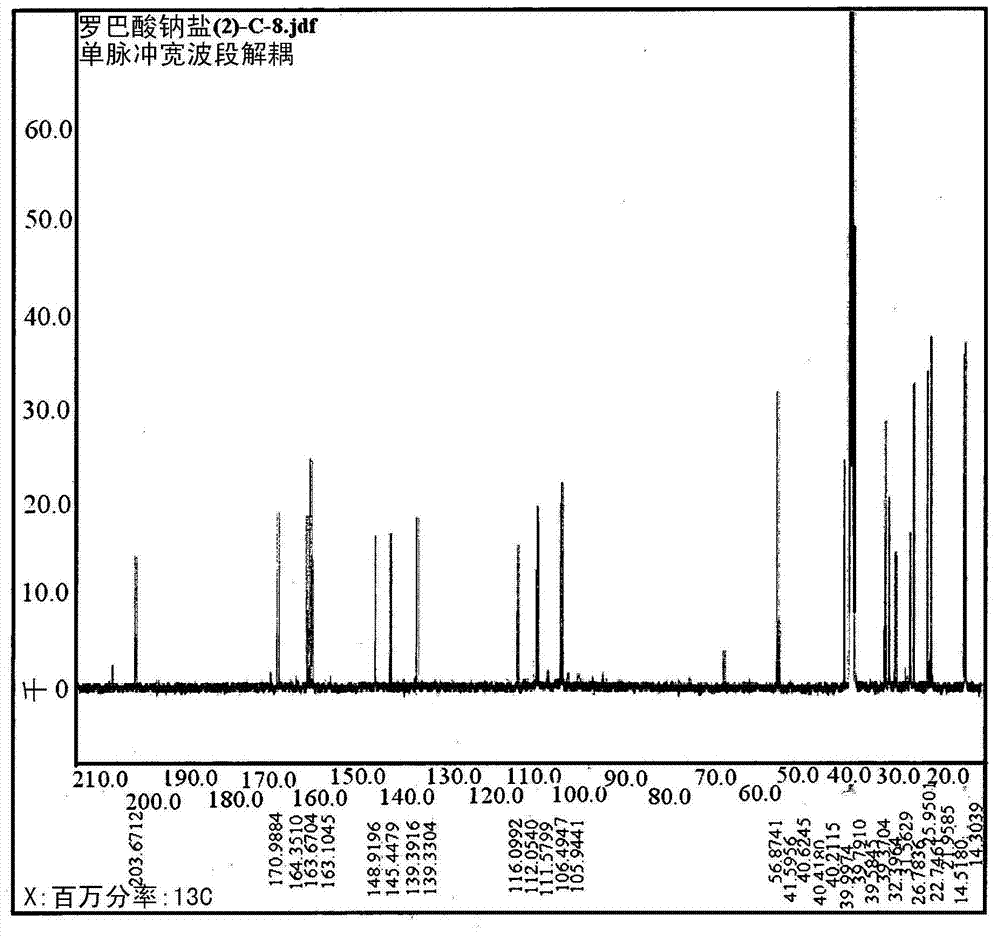
[0245] 2-2: Structural analysis of sodium robalate
[0246] The structure of sodium lobalate was confirmed by comparing the NMR data of the compound with that of lobalic acid. Dissolve each sample in DMSO-d 6 After the solvent, using a JEOL ECP-400 spectrometer (JEOL, Japan), each NMR data was measured, with DMSO-...
Embodiment 3
[0256] Analysis of protein tyrosine phosphatase-1b inhibitory activity of sodium robalate
[0257] To analyze protein tyrosine phosphatase-1b (PTP-1b) inhibitory activity, enzyme activity was measured spectroscopically.
[0258] That is, protein tyrosine phosphatase-1b (Bioneer Company, Korea) and protein tyrosine phosphatase-1b buffer (20mM Tris-HCl, pH8.0, 0.75mM sodium chloride , 0.5mM EDTA, 5mMβ-mercaptoethanol, 50% glycerol), add sodium robalate 0, 1, 3, 10, 30, 100, 300, 1000, 3000nM and matrix [pTyr1146] insulin receptor (Insulin Receptor ) (1142-1153, Sigma Company, U.S.), react at normal temperature for 10 minutes-30 minutes, then, add malachite green (Malachite Green)-molybdate dye solution (Molybdate Dye Solution) (1142-1153, Sigma Company , USA), and reacted at room temperature for 10 minutes, after finishing the reaction with protein tyrosine phosphatase-1b, sodium robalate and substrate, the absorbance was measured at 620nm.
[0259] results, such as Figure 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com