Moxifloxacin dispersible tablet and preparation method thereof
A technology of moxifloxacin hydrochloride and dispersible tablets, applied in the field of chemical pharmacy, can solve the problems of inconvenience for patients to take, less adverse reactions, etc., and achieve rapid drug dissolution, short disintegration time, good fluidity and compressibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-12
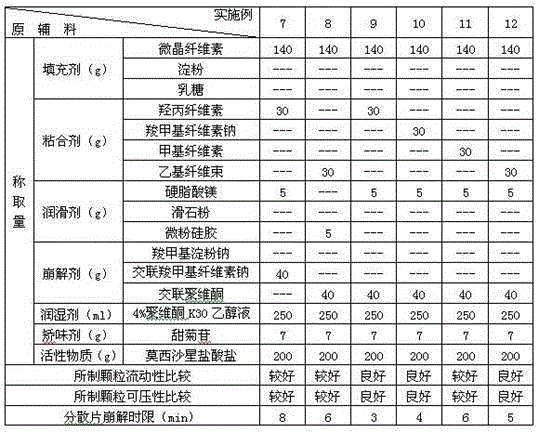
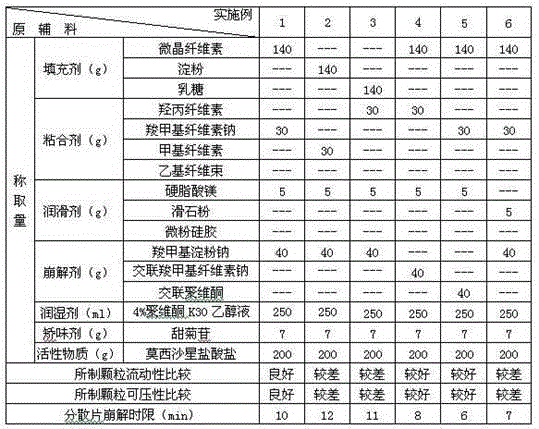
[0031] Test the selection of various excipients in the prescription, take each component according to the amount in Table 1 and 2, pass moxifloxacin hydrochloride through an 80 mesh sieve, filler, disintegrant (accounting for 65% of the total disintegration dose), Adhesives, flavoring agents, etc. are passed through a 60-mesh sieve, and the above components are mixed evenly. Subsequently, with an appropriate amount of 4% povidone K 30 The ethanol solution is prepared into a soft material, granulated through a 30-mesh sieve, dried at 55°C, and then granulated through a 24-mesh sieve. Then add a lubricant and a disintegrant (accounting for 35% of the total disintegrating dose), mix evenly and press into tablets to prepare dispersible tablets each containing 200 mg of moxifloxacin hydrochloride.
[0032] Table 1 The composition and investigation results of each component in Examples 1-6
[0033]
[0034] Table 2 The composition and investigation results of each component in ...
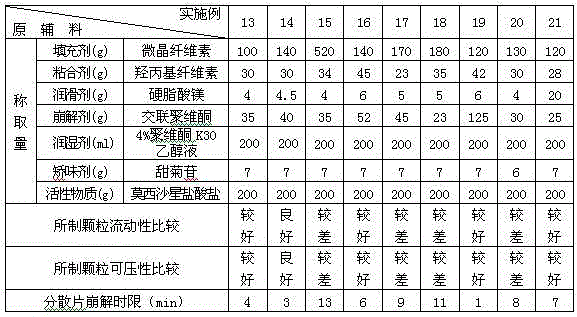
Embodiment 13-21
[0038] According to the preferred auxiliary materials in Example 9 above, the proportions of the various components of the auxiliary materials were studied in Examples 13-21, and each component was measured according to Table 3. Pass moxifloxacin hydrochloride through a 80-mesh sieve, microcrystalline cellulose, crospovidone (accounting for 65% of the total), hydroxypropyl cellulose, stevioside, etc. through a 60-mesh sieve, and mix the above components evenly . Subsequently, with an appropriate amount of 4% povidone K 30 The ethanol solution is prepared into a soft material, granulated through a 30-mesh sieve, dried at 55°C, and then granulated through a 24-mesh sieve. Magnesium stearate and crospovidone (accounting for 35% of the total amount) were then added, mixed uniformly and pressed into tablets to prepare dispersible tablets each containing 200 mg of moxifloxacin hydrochloride.
[0039] Table 3 Embodiment 13-21 each component weight composition and investigation resu...
Embodiment 22
[0043] Prepare each dispersible tablet containing 50mg of moxifloxacin hydrochloride active substance, each active substance content is about 33%, take each component according to the following weight:
[0044] Moxifloxacin hydrochloride 50g
[0045] Crospovidone 20g
[0046] Microcrystalline Cellulose 50g
[0047] Hypromellose 23g
[0048] Magnesium Stearate 3g
[0049] Stevioside 4g
[0050] 4% Povidone K 30 Proper amount of ethanol solution
[0051] Pass 50 g of the above-mentioned moxifloxacin hydrochloride through a 80-mesh sieve, 13 g of crospovidone, 50 g of microcrystalline cellulose, 23 g of hydroxypropyl cellulose, and 4 g of stevioside through a 60-mesh sieve, and mix the above components evenly. Subsequently, with an appropriate amount of 4% povidone K 30 The ethanol solution is prepared into a soft material, granulated through a 30-mesh sieve, dried at 55°C, and then granulated through a 24-mesh sieve. Then 7 g of crospovidone and 3 g of magnesium stearate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com