Device for electrolytically depositing nickel or cobalt
An electrowinning and electrowinning cell technology, which is applied in the field of electrolytic deposition equipment, achieves the effects of low electrical energy consumption, shortening the same-pole spacing, and saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
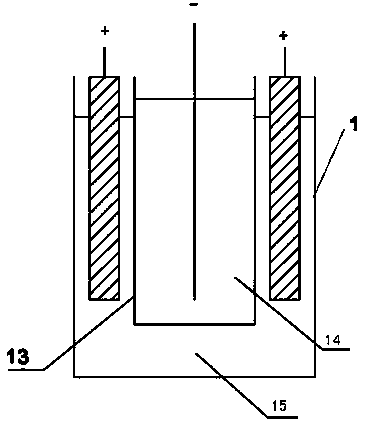
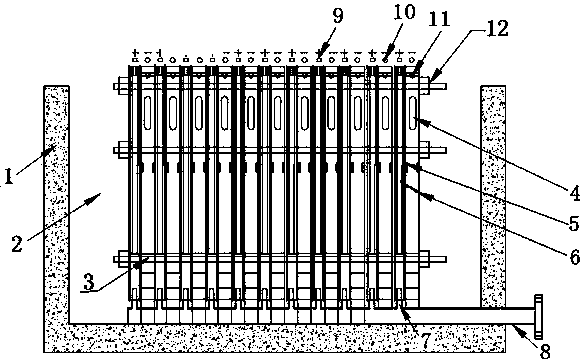
[0022] As shown in the figure, the device for electrowinning nickel or cobalt includes an electrowinning cell with a cathode frame and an anode frame. In the electrowinning cell, the number of cathode frames is one less than the number of anode frames. In the electrowinning cell One end is provided with a high-level tank, and the electrolyte in the high-level tank enters the electrowinning cell through the pipeline. Since the catholyte inlet is set on the cathode frame, the electrolyte in the electrowinning cell enters the cathode frame through the catholyte inlet. A permeable membrane with appropriate permeability is set between the anode frame and the anode frame, so the electrolyte enters the anode frame at a constant speed through the permeable membrane to complete the entire electrowinning process. , the acid mist discharge outlet is set on the upper end of the anode frame, so the anolyte produced is discharged out of the electrowinning cell through the anolyte outlet, and...
Embodiment 2
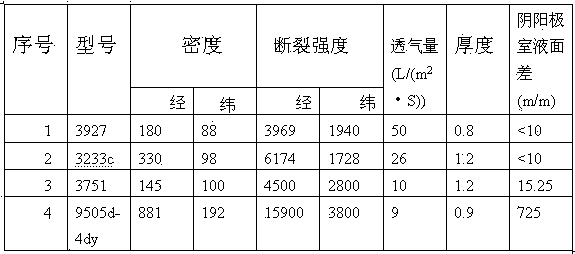
[0026] Adopt the electrowinning cell structure in embodiment 1, keep consistent with the size and the length of the electrowinning cell in the comparative example, set the quantity of the cathode sheet in the cathode frame in the electrowinning cell to be 34, and the pole spacing is 100mm, adopts model as 3751 polyester cloth is used as the permeable membrane between the cathode frame and the anode frame, the liquid level difference between the liquid level in the cathode frame and the liquid level in the anode frame is kept at 18 mm, the cell voltage is 3.0 / V, and the output is 0.454 t / slot·day, DC power consumption is 4050Kw·h.
Embodiment 3
[0028] The electrowinning cell structure in Example 1 is adopted, and the size and length of the electrowinning cell in the comparative example are kept consistent. The number of cathode sheets in the cathode frame in the electrowinning cell is set to 48, the pole spacing is 70mm, and the model is 3751. The polyester cloth is used as the permeable membrane between the cathode frame and the anode frame, and the liquid level difference between the liquid level in the cathode frame and the liquid level in the anode frame is kept at 20mm, and the obtained cell voltage is 2.5 / V, and the output It is 0.655t / slot·day, and the DC power consumption is 3645Kw·h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com