Golden phosphorous acetyletic compound, and preparation method and applications thereof
A compound, gold phosphoryne technology, applied in the field of new gold phosphoryne compounds, can solve the problem of loss of biological activity, and achieve the effect of good tumor inhibition, wide application prospect and strong stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 The preparation of the gold phosphyne compound of the present invention (the R structure is p-methoxyphenyl, and the R' structure is 2-thiophene diphenylphosphine)
[0043] Add chloroauric acid (800mg, 1.94mmol), 1.12g potassium bromide, 4mL water into a 50mL two-necked flask, stir to dissolve it, add 14mL of acetone-water (1:1) solution, the solution of the reaction system is dark red. In an ice bath, sulfur dioxide was slowly introduced until the color of the reaction system turned light yellow.
[0044] Add 0.8mL of acetone solution dissolved with p-methoxyphenyl alkyne (2.04mmol), 1.6g of sodium acetate, and then react at room temperature for 1h. A large amount of yellow solids are formed. After the reaction is completed, filter with suction, and filter the cake with acetone and Wash with ether.
[0045] Add 0.1mmol of the gold alkyne compound prepared above, 0.1mmol of 2-thiophene diphenylphosphine ligand, and 2ml of dichloromethane into a 15mL press...
Embodiment 2
[0050] Embodiment 2 The preparation of the aurophosphyne compound of the present invention (the R structure is phenyl, and the R' structure is 2-thiophene diphenylphosphine)
[0051] Add chloroauric acid (800mg, 1.94mmol), 1.12g potassium bromide, 4mL water into a 50mL two-necked bottle, stir to dissolve, add 14mL of acetone-water (1:1) solution, the solution of the reaction system is dark red. In an ice bath, sulfur dioxide was slowly introduced until the color of the reaction system turned light yellow.
[0052] Add 0.8mL of acetone solution dissolved in phenylyne (2.04mmol), 1.6g of sodium acetate, and then react at room temperature for 1h, a large amount of yellow solid is formed, after the reaction is completed, filter with suction, and wash the filter cake with acetone and ether respectively.
[0053] Add 0.1mmol of the gold alkyne compound prepared above, 0.1mmol of 2-thiophene diphenylphosphine ligand, and 2ml of dichloromethane into a 15mL pressure-resistant tube, re...
Embodiment 3
[0058] The stability test of embodiment 3 gold phosphyne compound of the present invention
[0059] The stability test program is as follows:
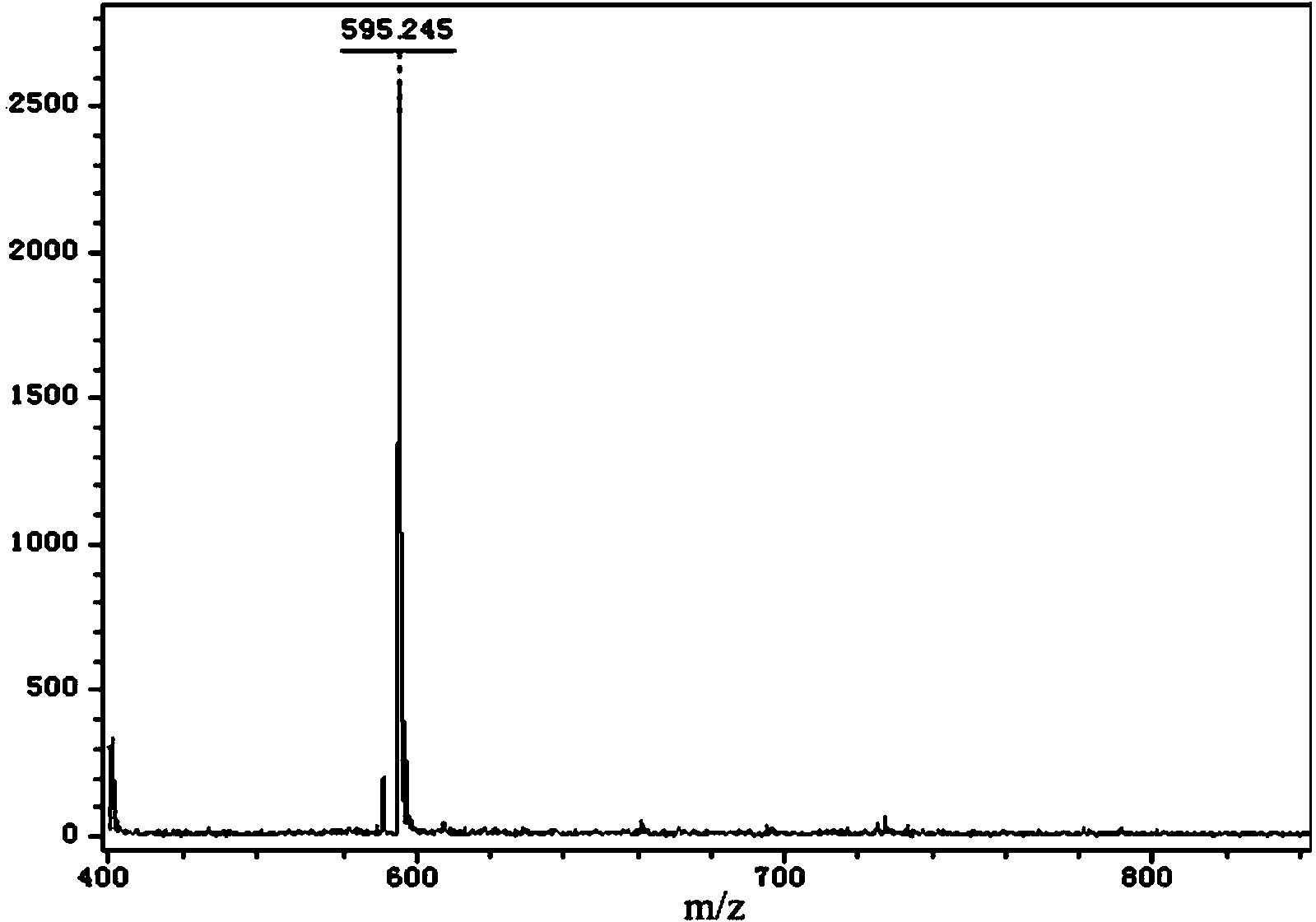
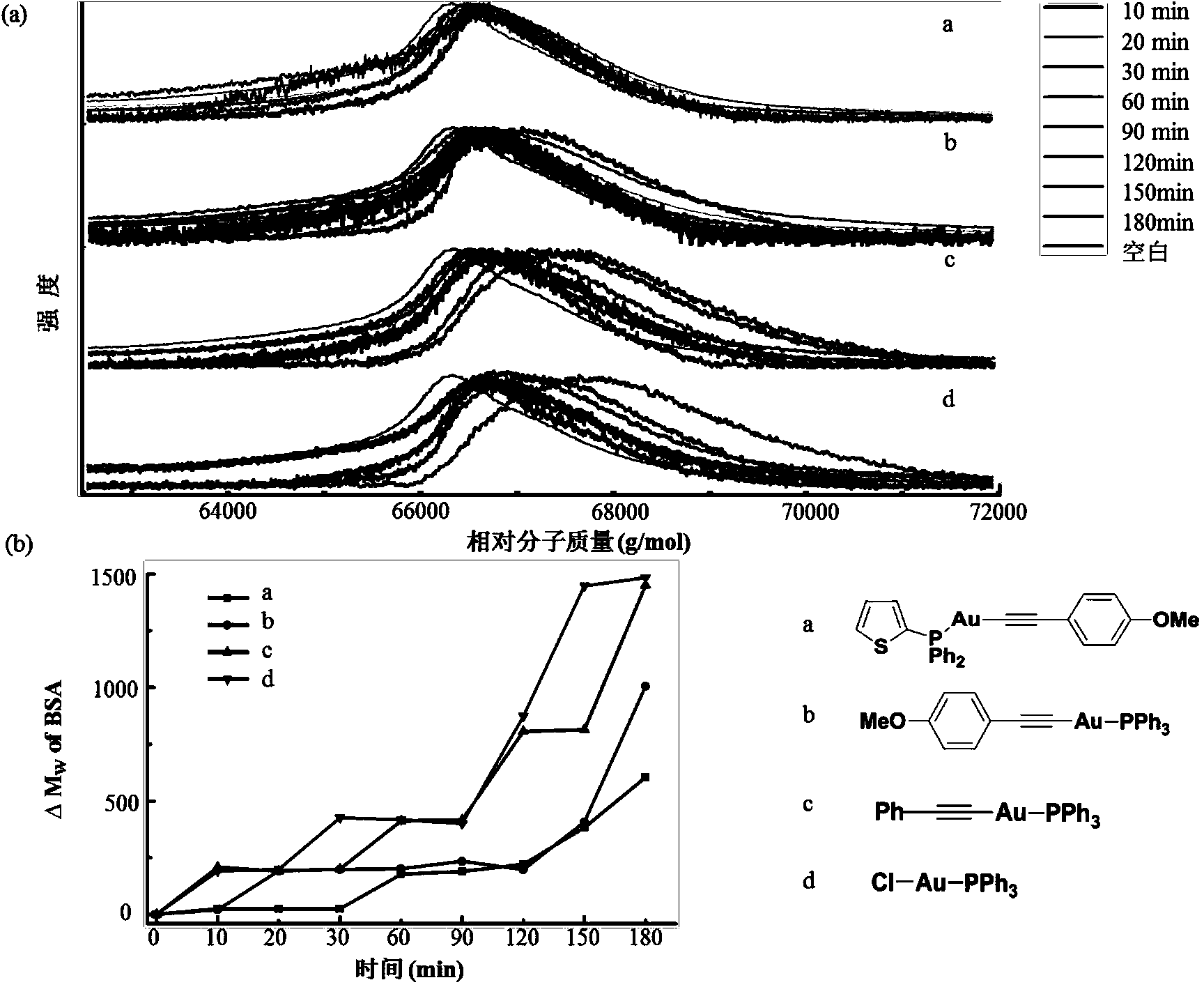
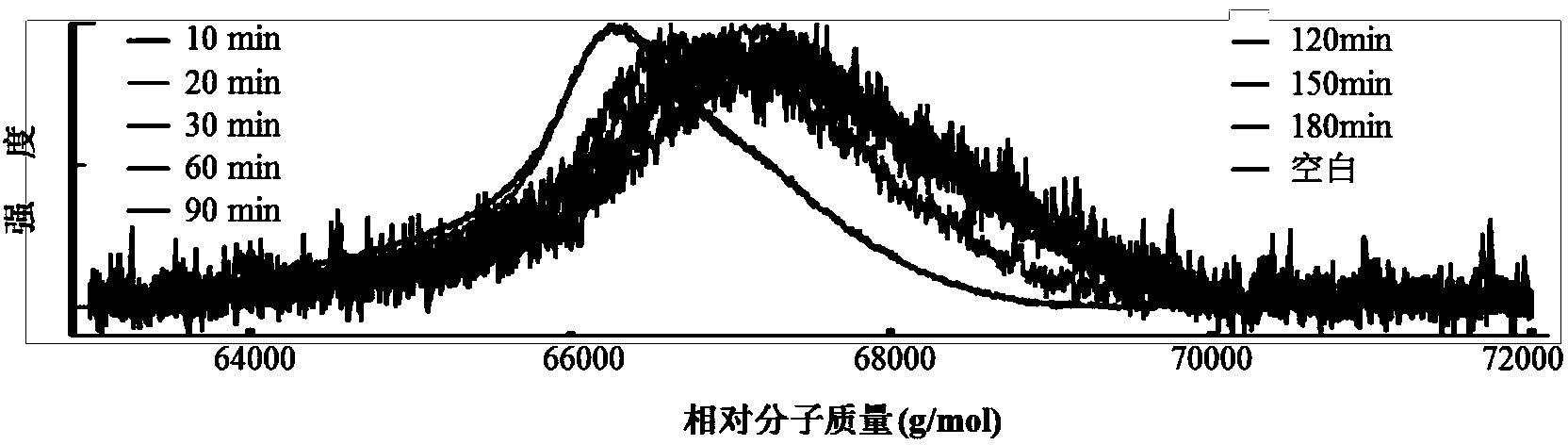
[0060] Dissolve 6 mg of bovine serum albumin (BSA) in 0.6 mL of water as a stock solution. Add 20 μM of the auropharyne compound prepared in Example 1 and Example 2 and the other three as controls to the 10-fold diluted BSA solution, take 2 μL of the solution every 30 minutes, and use matrix-assisted laser desorption ionization time-of-flight mass spectrometry to test The molecular weight of BSA protein changes with time; the stability of the compound is verified by the strength of the ligand exchange between the gold phosphyne compound and BSA. The result is as figure 2 , image 3 shown.
[0061] figure 2 The results show that: a is the aureophosphyne compound prepared in Example 1, and b, c, and d are three kinds of aureophosphyne compounds used as controls. Compared with b, c, and d reference compounds, the BSA molecular wei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com