A kind of preparation method of Axitinib intermediate and its application in the preparation of Axitinib
A technology of axitinib and intermediates, applied in the field of pharmaceutical chemical synthesis, can solve the problems of new impurities and incomplete reactions, and achieve the effects of reducing reaction steps, reducing costs, and high product yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
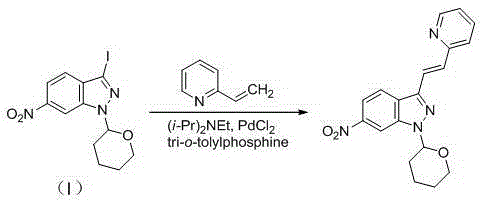
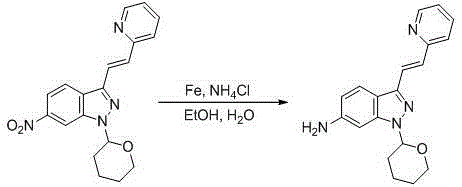
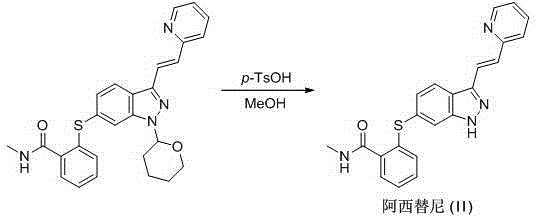
[0053]A kind of preparation method of Axitinib intermediate, comprises the following steps:
[0054] (1) Synthesis of 6-nitro-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole
[0055] Add acetonitrile (2L) to a 5L reaction flask, then add 6-nitroindazole (163.1g, 1.0mol), 3,4-dihydro-2H-pyran (168.2g, 2.0mol), 2,3- Dichloro-5,6-dicyano-p-benzoquinone (22.7g, 0.1mol), heated to 82°C and refluxed for 2 hours until the reaction was complete, cooled to room temperature, rotary evaporated to dryness, added 2L of dichloromethane and 2L of water, Stir for 1 hour, separate layers, wash the organic phase with brine, dry over anhydrous sodium sulfate, filter, and rotary evaporate to dryness, then dissolve in 2 L of acetonitrile, freeze in an ice-salt bath to -5°C and stir for 2 hours, suction filter, filter cake Wash with a small amount of cold acetonitrile, recrystallize from ethanol, and dry under vacuum at 60°C for 12 hours to obtain an off-white solid, 6-nitro-1-(tetrahydro-2H-pyran-2-yl)-...
Embodiment 2
[0085] A kind of preparation method of Axitinib intermediate, comprises the following steps:
[0086] (1) Synthesis of 6-nitro-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole
[0087] Add ethyl acetate (2L) to a 5L reaction flask, then add 6-nitroindazole (163.14g, 1.0mol), 3,4-dihydro-2H-pyran (210.3g, 2.5mol), p-toluene Sulfonic acid (20.7g, 0.12mol), heat up to 78°C and reflux for 3 hours until the reaction is complete, cool to room temperature, rotary evaporate to dryness, add 2L of dichloromethane and 2L of water, stir for 1 hour, separate layers, and use brine for the organic phase washed, dried over anhydrous sodium sulfate, filtered, and rotary evaporated to dryness, then dissolved in 2L of acetonitrile, frozen in an ice-salt bath to -5°C and stirred for 2 hours, filtered with suction, washed with a small amount of cold acetonitrile, recrystallized from ethanol, 60 After vacuum drying at ℃ for 12 hours, 223.3 g of off-white solid was obtained, 6-nitro-1-(tetrahydro-2H-pyran-...
Embodiment 3
[0117] A kind of preparation method of Axitinib intermediate, comprises the following steps:
[0118] (1) Synthesis of 6-nitro-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole
[0119] Add toluene (2L) to a 5L reaction flask, then add 6-nitroindazole (163.1g, 1.0mol), 3,4-dihydro-2H-pyran (193.5g, 2.3mol), methanesulfonic acid ( 14.4g, 0.15mol), heated to 85°C and refluxed for 3.5 hours until the reaction was complete, cooled to room temperature, rotary evaporated to dryness, added 2L of dichloromethane and 2L of water, stirred for 1 hour, separated, washed the organic phase with brine, Dry over anhydrous sodium sulfate, filter, rotary evaporate to dryness, then dissolve in 2L of acetonitrile, freeze in an ice-salt bath to -5°C and stir for 2 hours, filter with suction, wash the filter cake with a small amount of cold acetonitrile, recrystallize with ethanol, vacuum at 60°C After drying for 12 hours, an off-white solid was obtained, 6-nitro-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole, 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com