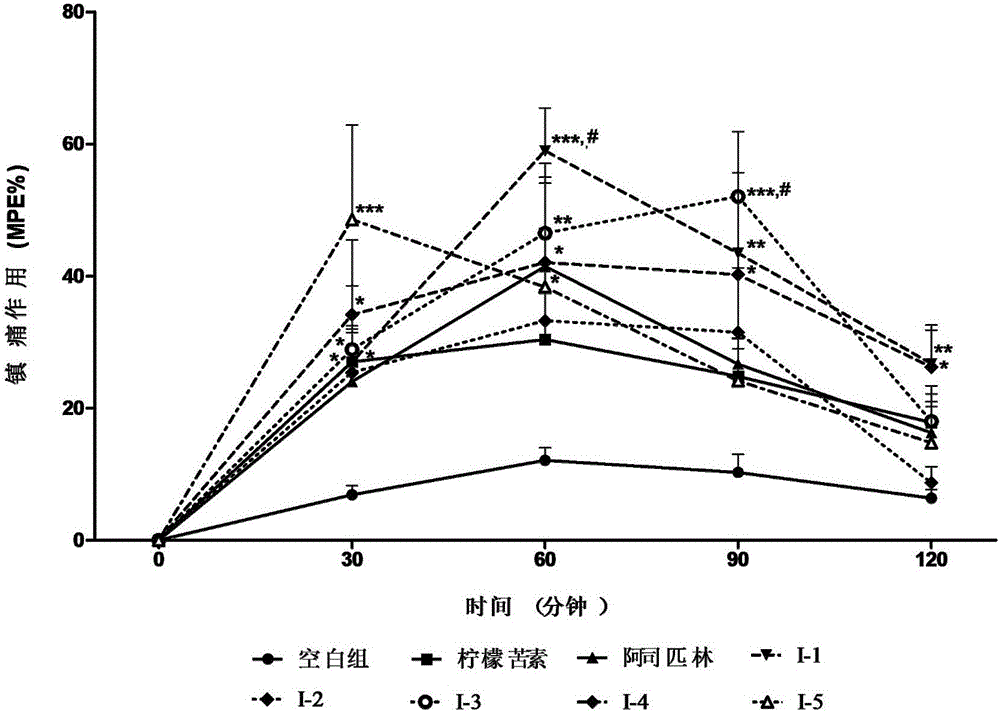
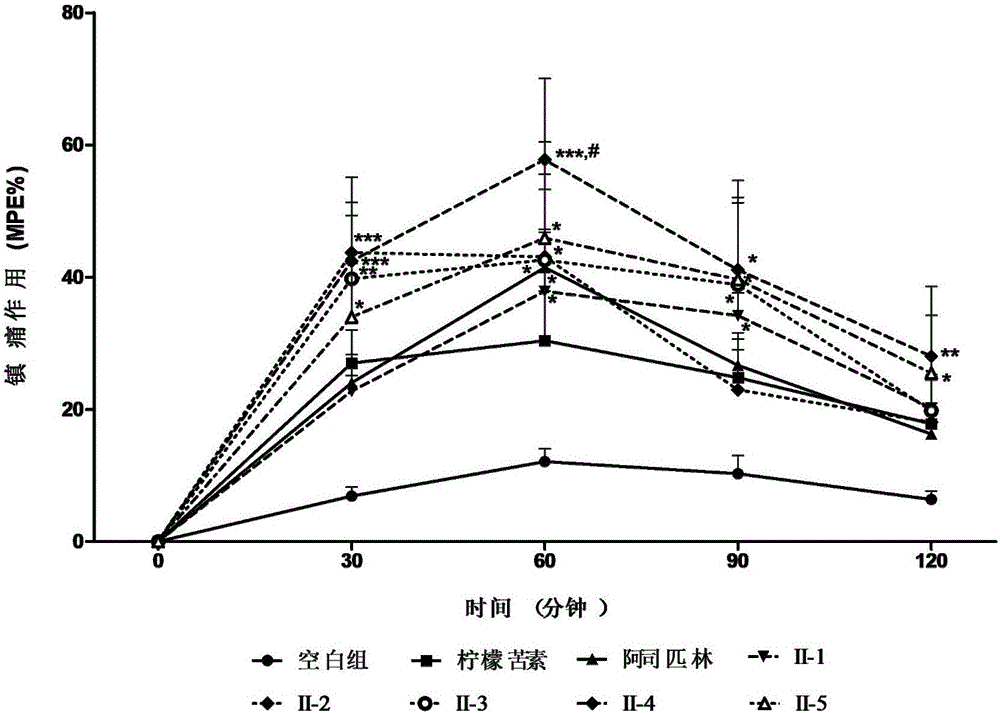
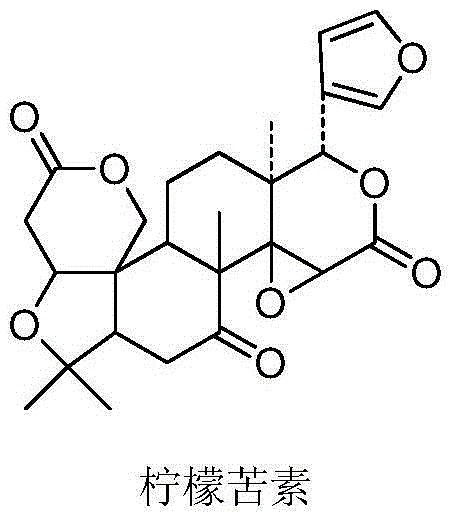
Limonin oxime ether derivative, its preparation method and medical application
A use and pharmaceutical technology, applied in the fields of water-soluble limonin and deoxylimonin oxime ether derivatives, anti-inflammatory effects, and analgesia, can solve the problems of low oral bioavailability and limited clinical application, and achieve good Analgesic and pain relief effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Preparation of Compound IV
[0059] Add 1.0g (0.002mol) of limonin (III), 1.1g (0.016mol) of hydroxylamine hydrochloride, 45ml of absolute ethanol, and 15ml of pyridine into a 100ml eggplant-shaped bottle, and heat to reflux for 2.5h. Thin-layer chromatography (TLC) detection, after the reaction is complete, the reaction solution is cooled to room temperature, poured into 125ml of 5% dilute hydrochloric acid prepared in advance, adjusted to acidic pH, and cooled with ice water. The aqueous layer was extracted three times with 50 ml of dichloromethane, the combined extracts were washed three times with saturated brine, and dried over anhydrous sodium sulfate. Filter and distill off the solvent under reduced pressure. The crude product was subjected to column chromatography with dichloromethane:methanol (125:1) to obtain 0.98 g of a white solid with a yield of 95%. mp>250℃; 1 HNMR (CDCl 3 ,500MHz),δ(ppm):7.70(1H,brs),7.39(2H,d,J=1.7Hz),6.34(1H,s),5.46(1H,s),4.68(1H,d,...
Embodiment 2
[0061] Preparation of Compound I-1
[0062] Completely dissolve 0.50 g (0.001 mol) of IV in 25 ml of anhydrous tetrahydrofuran in a 50 ml eggplant-shaped bottle. Now add 0.2g (0.005mol) of 60% sodium hydride, stir at room temperature for 30min, add 0.55g (0.003mol) of 1-(2-chloroethyl) piperidine hydrochloride, potassium iodide 0.17 (0.001mol) and catalytic amount of TBAB, after continuing to stir at room temperature for 1 h, the temperature was raised to 80° C. for 12 h. It was detected by TLC that the reaction was complete, cooled, and the solvent was evaporated under reduced pressure. Add 100ml of water to the residue, adjust the pH to acidic with 5% dilute hydrochloric acid, extract the aqueous layer three times with 70ml of dichloromethane, combine the extracts, wash with saturated brine three times, and dry over anhydrous sodium sulfate. Filter and distill off the solvent under reduced pressure. The crude product was subjected to column chromatography with dichloromet...
Embodiment 3
[0065] Preparation of Compound I-2
[0066] Using compound IV and 1-(2-chloroethyl)morpholine hydrochloride as raw materials, the operation is the same as that of I-1, and the crude product is chromatographed with dichloromethane:methanol (75:1) to obtain I-2 white Solid 0.29g, yield 48%. mp138~141℃;IR(KBr,υcm -1 ):2963,1750,1025,807; 1 H NMR (CDCl 3 ,500MHz),δ(ppm):7.41(2H,d,J=4.6Hz),6.36(1H,s),5.49(1H,s),4.66(1H,d,J=13.0Hz),4.33(1H ,d,J=13.0Hz),3.96(1H,brs),3.86(4H,brs),3.80(1H,s),3.45(1H,dd,J 1 =2.9Hz,J 2 =14.0Hz),2.95(1H,dd,J 1 =3.8Hz,J 2 =16.8Hz), 2.67–2.93(4H,brs), 2.66(1H,dd,J 1 =1.9Hz,J 2 =16.7Hz),2.39(1H,d,J=11.6Hz),1.73-2.06(9H,m),1.49-1.53(2H,m),1.30(3H,s),1.25(3H,s),1.17 (3H,s),0.97(3H,s); 13 C NMR (CDCl 3 ,300MHz),δ(ppm):169.37,166.96,158.44,143.20,140.98,120.14,109.69,80.35,79.27,78.28,71.86,66.88,65.76,64.73,60.65,5546,54.247,46.9 ,37.99,35.84,33.09,30.27,21.59,21.34,19.78,19.57,17.99;HR-ESIMS m / z599.2958[M+H] + (calcd for C 32 h 43 N 2 o 9 ,59...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com