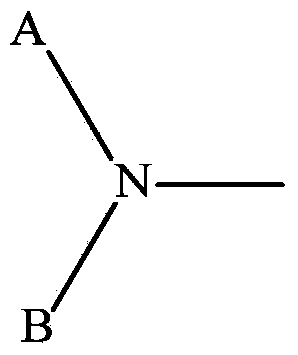
Use of cxcr4 antagonists
An antagonist, methylene technology, applied in the field of use of CXCR4 antagonists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
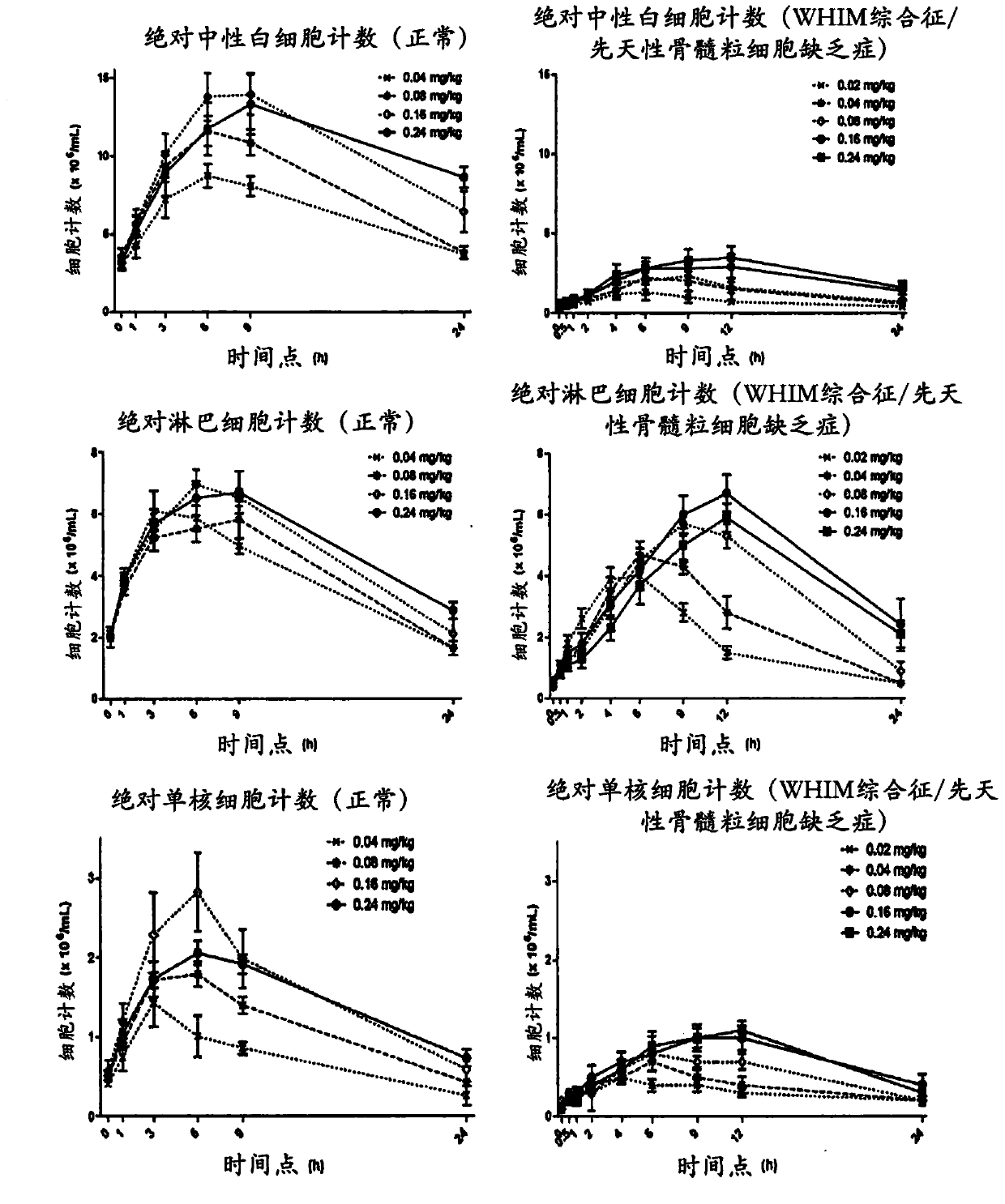
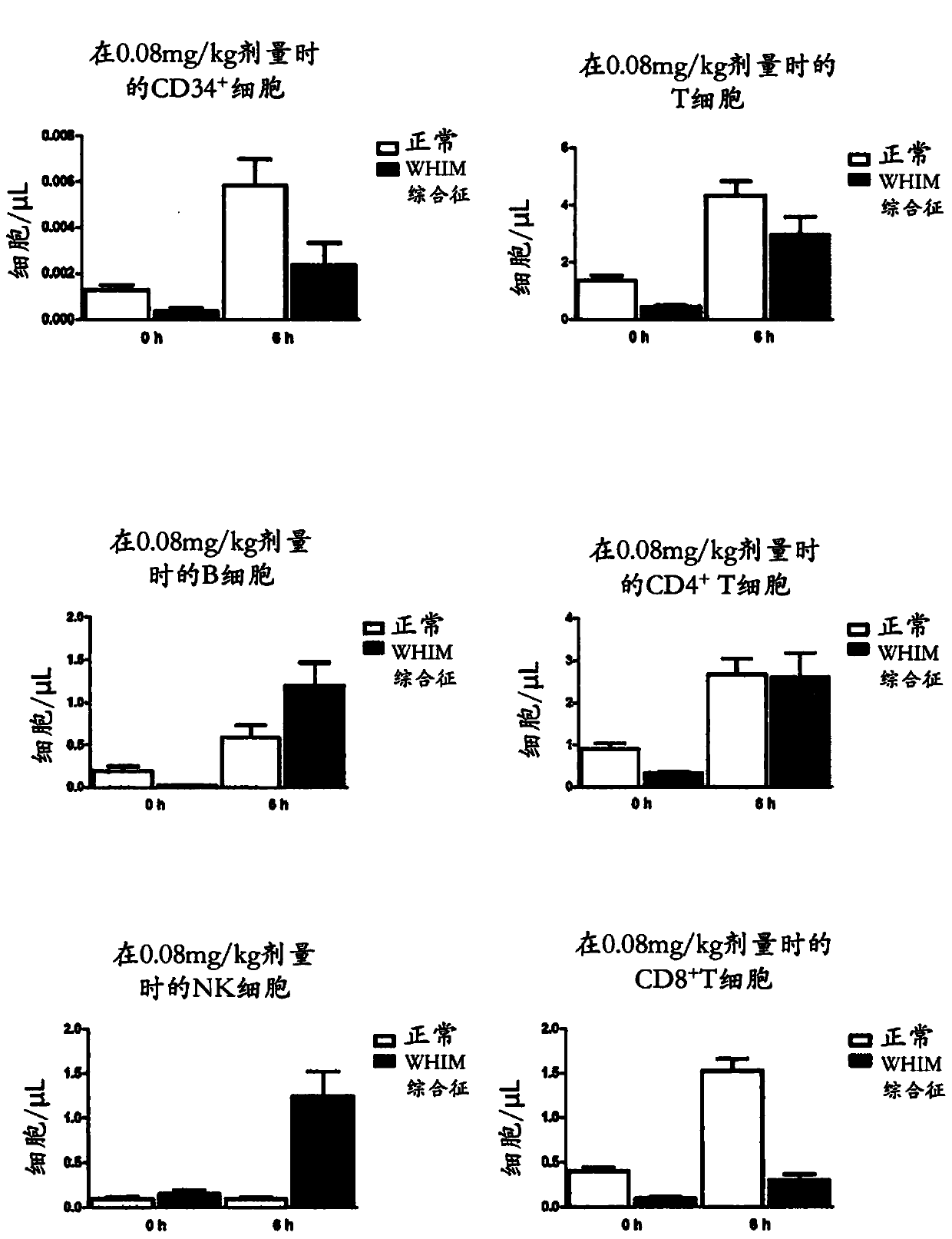
[0389] Example 1: Clinical Trial Results - Plerixafor is a potential therapy for congenital myeloid agranulocytosis, WHIM syndrome.
[0390] Research design: This is an open-label, single-center, phase I study to examine the hematological effects, pharmacokinetics, and safety of plerixafor in patients with congenital myelogranulocytosis due to CXCR4 mutations. The study used serial, escalating doses of plerixafor administered on days 1, 3, 5, 8, and 10. Five escalating dose levels, 20mcg / kg, 40mcg / kg, 80mcg / kg and 240mcg / kg, were examined daily in patients. Subjects were patients at the University of Washington Comprehensive Clinical Research Center for up to 10 days; the study required subject participation for up to 14 days. Patients were monitored for the hematological effects of plerixafor and observed for adverse reactions. Plerixafor will be discontinued if a normal blood neutrophil count is achieved and maintained for at least 24 hours prior to the highest dose.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com