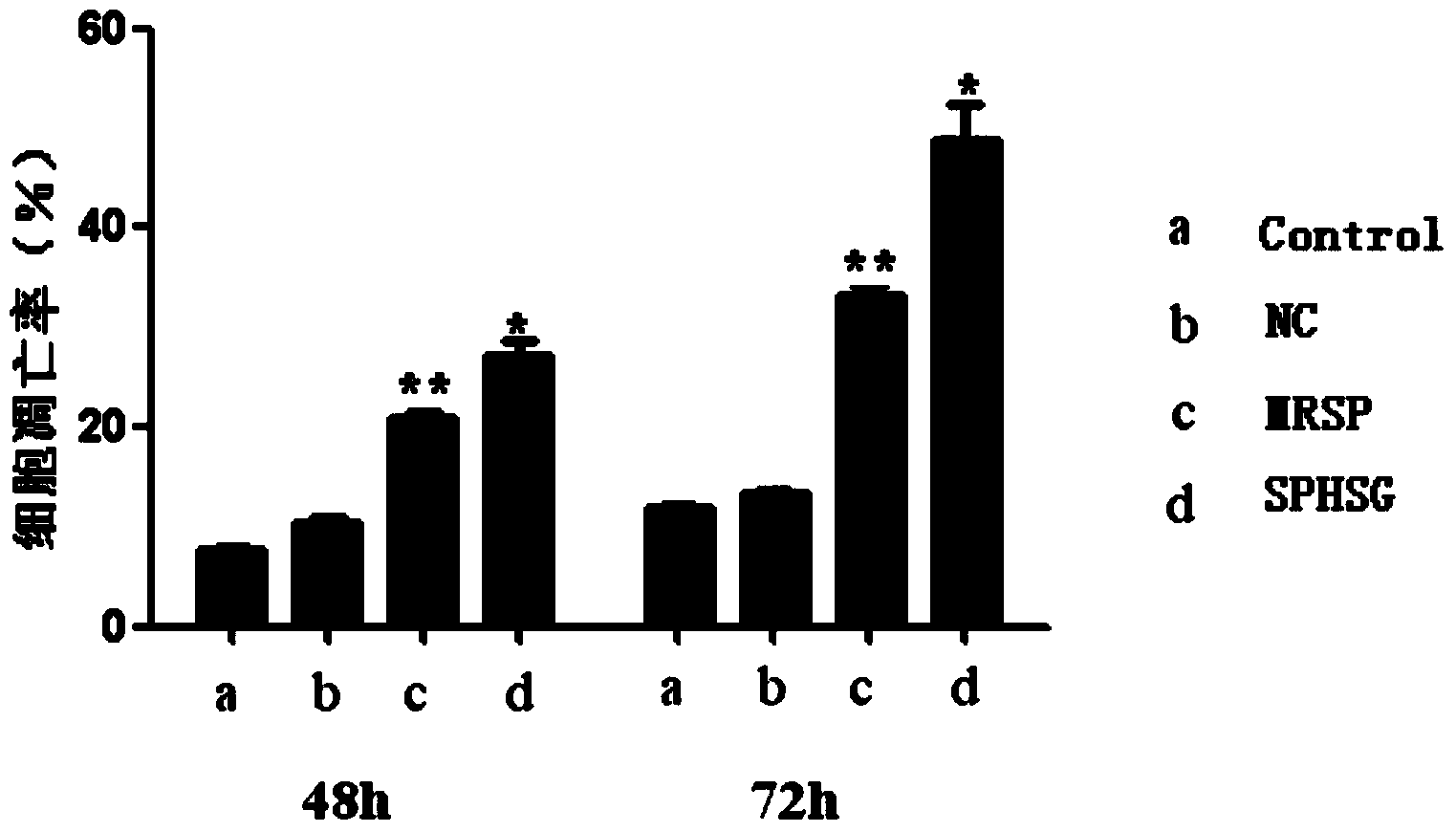Polypeptide for inhibiting cell proliferation and inducing apoptosis and preparation method and use thereof
A technique for inhibiting cell and apoptosis, applied in the field of polypeptides that inhibit cell proliferation and induce apoptosis and its preparation, achieve great clinical application value, induce apoptosis, and have obvious effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experiment example 1
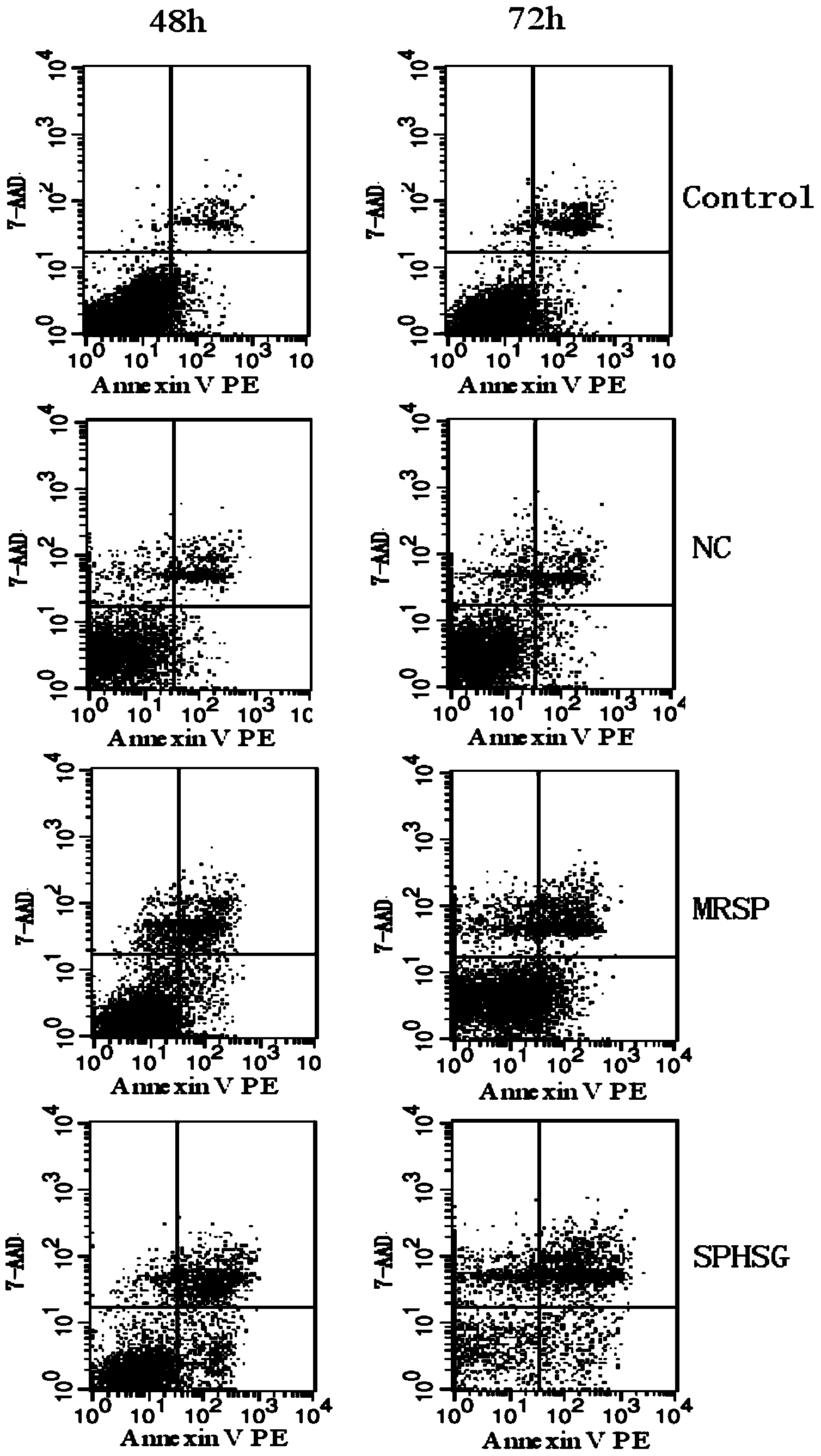
[0056] Experimental Example 1: Detection of smooth muscle cell apoptosis by flow cytometry
[0057] 1. Take the rat VSMCs of the 4th-9th generation, and count them accurately according to 5×10 5 Cells / well were seeded in a six-well plate, and 2ml of DMEM (dulbecco's modified eagle medium cell culture medium) high-glucose medium was added. Three replicate wells could be set up for each group, and the cells were incubated at 37°C and 5% CO 2 Continue culturing for 24 hours in a cell culture incubator.
[0058] 2. Synchronize, discard the medium in each experimental well, wash with sterile PBS (phosphate buffer solution) twice, pay attention to the operation should be gentle, try to avoid blowing up the cells, and then use a pipette gun to respectively Add 2ml of serum-free DMEM medium to each experimental well. Add SPHSG, MRSP and NC (invalid control) to each experimental group at a concentration of 25 μmol / L, and only add serum-free medium to the blank group, mix well and pla...
experiment example 2
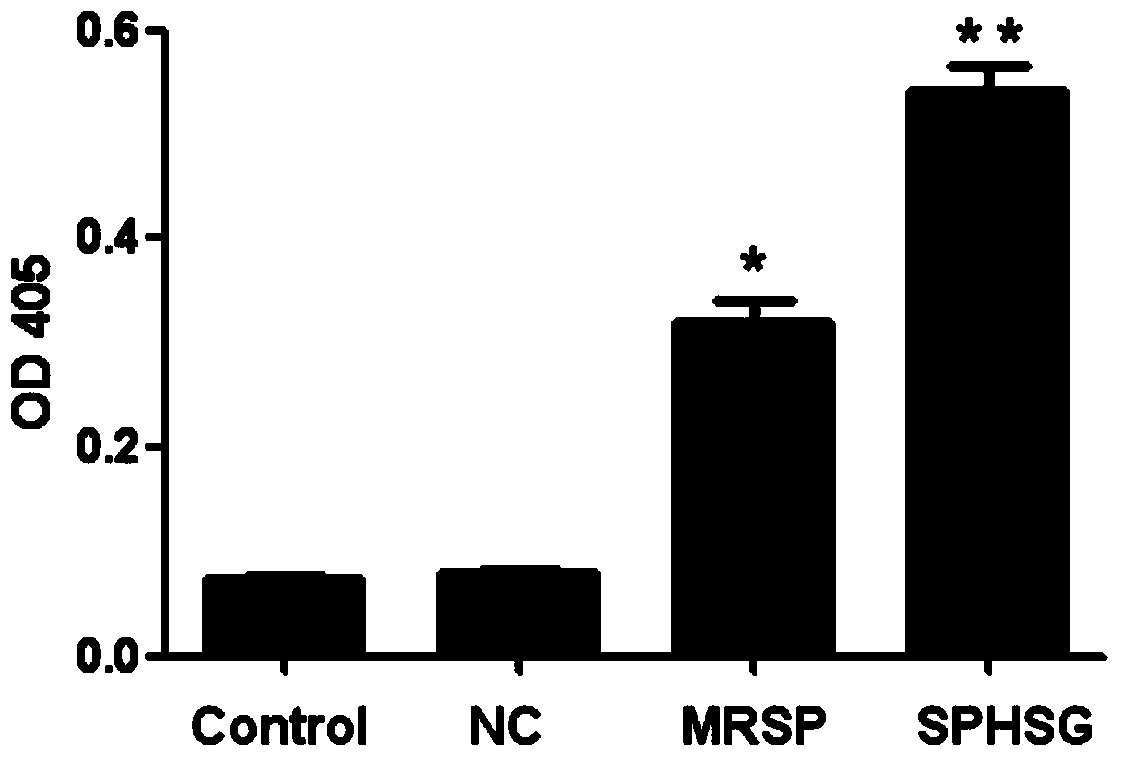
[0064] Experimental Example 2: Detection of Caspase3 Enzyme Activity
[0065] 1. Take the rat VSMCs of the 4th-9th generation, and count them accurately according to 1×10 6 Seed each cell / well in a 60mm cell culture dish, add 3ml DMEM high-glucose medium, set up 3 replicate wells for each group, and incubate at 37°C, 5% CO 2 Continue culturing for 24 hours in a cell culture incubator.
[0066] 2. Synchronize, discard the medium in each experimental well, wash with sterile PBS twice, pay attention to the operation to be gentle, try to avoid blowing up the cells, and then add 3ml serum-free DMEM to each experimental well with a pipette gun Medium. SPHSG, MRSP and NC were added to each experimental group at a concentration of 25 μmol / L, and only serum-free medium was added to the blank group, mixed evenly and placed in a cell culture incubator for 8 hours.
[0067] 3. Collect the cells when the cells have not entered the late stage of apoptosis, and collect the cells completel...
experiment example 3
[0076] Experimental example 3: In situ terminal transferase labeling technique (TUNEL) detection of apoptosis rate
[0077] 1. For cell slides, soak the autoclaved glass slides with 10% poly-slothine, air-dry them under sterile conditions, transfer them to a six-well plate, and set aside.
[0078] 2. Take the rat VSMCs of the 4th-9th generation, and count them accurately according to 2×10 5 Cells / well were inoculated in a six-well plate, 2ml DMEM high-glucose medium was added, and three replicate wells could be set up for each group, and the 2 Continue culturing for 24 hours in a cell culture incubator.
[0079] 3. Synchronize, suck up and discard the medium in each experimental well, wash with sterile PBS twice, pay attention to the operation to be gentle, try to avoid blowing up the cells, and then add 2ml serum-free DMEM to each experimental well with a pipette gun Medium. SPHSG, MRSP and NC were added to each experimental group at a concentration of 25 μmol / L, and only ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com