Application of kinesin and its homologues in the preparation of drugs for the treatment of diabetic complications
A technology of kelkinin and diabetes, applied in the field of medicine, to achieve the effects of less addiction, no tolerance and addiction, and less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
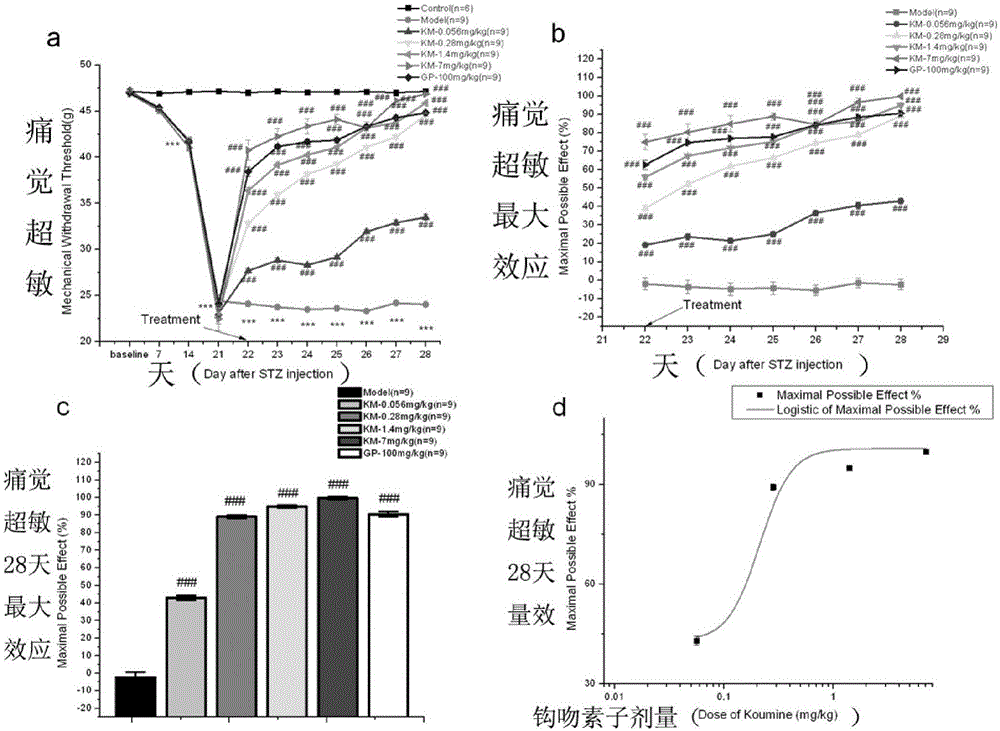
[0026] Improvement of hyperalgesia in diabetic rats by subcutaneous injection of kelkinin in the back of the neck.
[0027] 1.1 Drugs and reagents:
[0028] Gelkinin is extracted, separated and purified from the natural plant Gelkins by the applicant of the present invention, see Patent No.: 200810071469.9, the title of the invention is "Method for Separating and Preparing Gelkins Alkaloid Monomers from Gelkins by High-speed Countercurrent Chromatography" and Patent No. 2011101746576 The disclosed technology is the prior art with a purity of >98.5% (accurately weigh keloids, dissolve with 1mol / l HCl, dilute with normal saline, adjust pH with 1mol / l NaOH (4
[0029] 1.2 Animals:
[0030] SD rats, m...
Embodiment 2
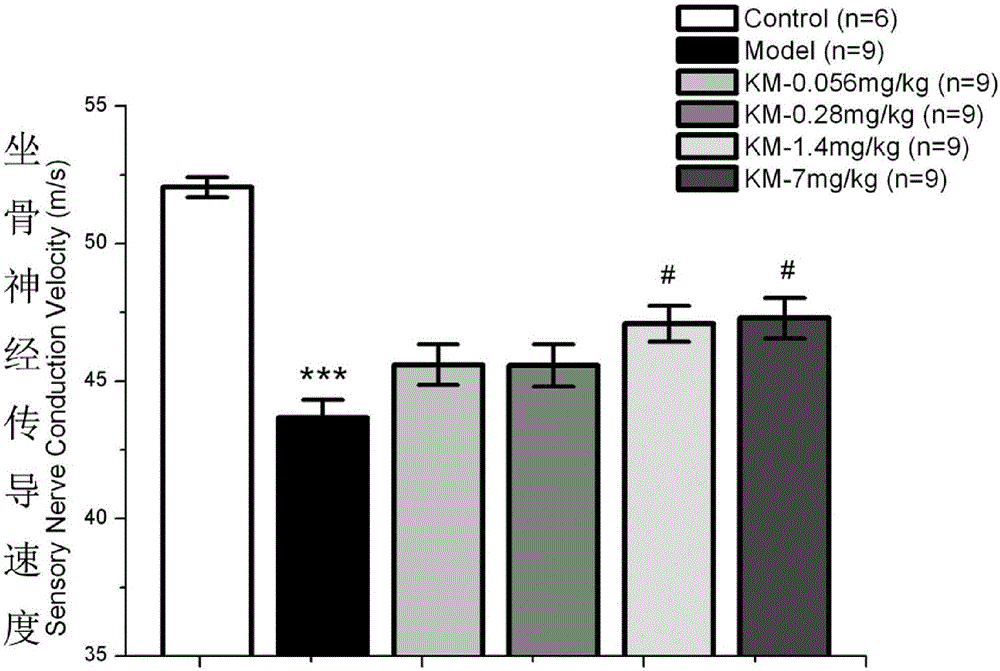
[0042] Improvement of nerve conduction impairment in diabetic rats by subcutaneous injection of kelezin in the back of the neck.
[0043] 2.1 Drugs and reagents:
[0044] Kiskinin is obtained by the applicant of the present invention by extracting, separating and purifying the natural plant Gelkins, with a purity of >98.5% (accurately weigh kelkinin, dissolve it with 1mol / l HCl, dilute with normal saline, adjust the pH with 1mol / lNaOH (4
[0045] 2.2 Animals:
[0046] SD rats, male, 180-220g, provided by Shanghai Slack Animal Center. They were raised at room temperature at 25°C under a light-dark cycle of 12 / 12h, free to eat and drink, and used for formal experiments after 3 days of adaptation to th...
Embodiment 3
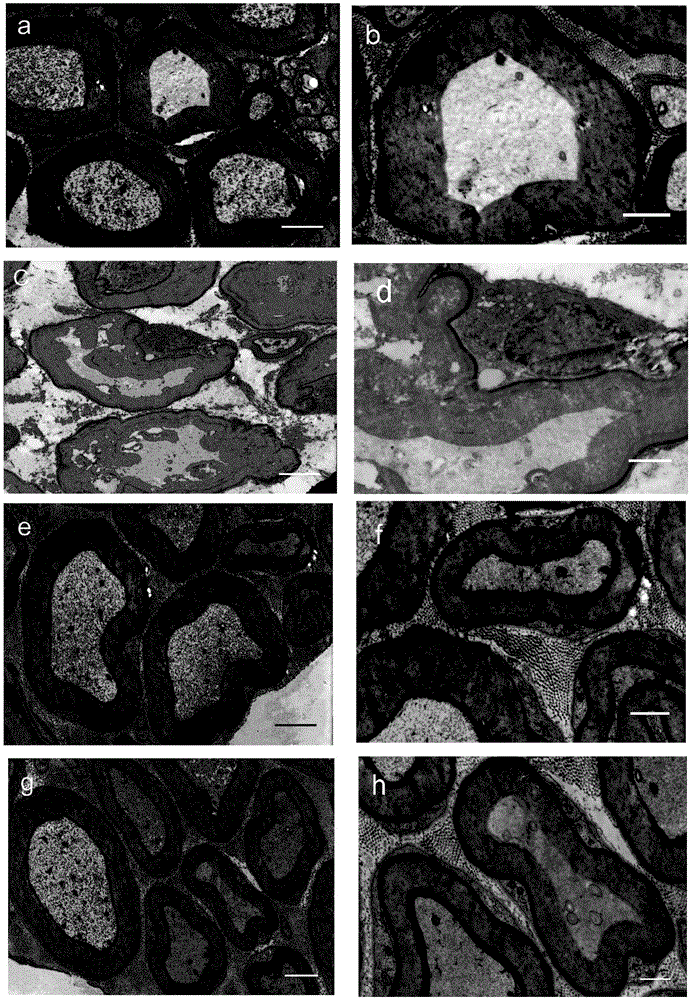
[0057] Improvement of neurofiber pathology in diabetic rats by subcutaneous injection of kelkinin in the back of the neck.
[0058] 3.1 Drugs and reagents:
[0059] Kiskinin is obtained by the applicant of the present invention by extracting, separating and purifying the natural plant Gelkins, with a purity of >98.5% (accurately weigh kelkinin, dissolve it with 1mol / l HCl, dilute with normal saline, adjust the pH with 1mol / lNaOH (4
[0060] 3.2 Animals:
[0061] SD rats, male, 180-220g, provided by Shanghai Slack Animal Center. They were raised at room temperature at 25°C under a light-dark cycle of 12 / 12h, free to eat and drink, and used for formal experiments after 3 days of adaptation to the labo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com