Pharmaceutical preparation for treating diabetes and preparation method thereof
A technology of drugs and compositions, applied in the field of polypeptide pharmaceutical preparations, can solve the problems of skin irritation and sensitization, and achieve the effects of convenient transportation and enhanced stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Investigating the dissolution of liraglutide powder under different pH conditions
[0048] Dissolve an appropriate amount of liraglutide original powder in water for injection and disodium hydrogen phosphate buffer with different pH, and the dissolution conditions are shown in Table 1:
[0049] Table 1 Dissolution of the original powder in different pH solutions
[0050] The concentration of liraglutide Dissolution Water for Injection 6mg / ml insoluble 10mM Disodium Hydrogen Phosphate Buffer (pH7.00) 6mg / ml insoluble 10mM Disodium Hydrogen Phosphate Buffer (pH7.50) 6mg / ml Colorless and clear 10mM Disodium Hydrogen Phosphate Buffer (pH8.00) 6mg / ml Colorless and clear 10mM Disodium Hydrogen Phosphate Buffer (pH8.15) 6mg / ml Colorless and clear 10mM Disodium Hydrogen Phosphate Buffer (pH8.50) 6mg / ml Colorless and clear 10mM Disodium Hydrogen Phosphate Buffer (pH9.00) 6mg / ml Colorless and cl...
Embodiment 2
[0052] Example 2 Investigate the osmotic pressure of solutions containing different osmotic pressure regulators
[0053] Dissolve different osmotic pressure regulators in 10mM disodium hydrogen phosphate buffer solution, add liraglutide powder (6mg / ml) while stirring, and then adjust the pH value to pH8.15 with sodium hydroxide. Finally, the above solutions were filtered with 0.22 μm filters, respectively. The test results of the concentration and osmotic pressure of each solution isotonic agent are shown in Table 2:
[0054] Table 2 Concentration and osmotic pressure test results of isotonic agent
[0055] Isotonic agent Osmolarity Negative control (no isotonic agent) 0.041 Methionine (15mg / ml) 0.141 Glycine (15mg / ml) 0.301 L-Arginine (25mg / ml) 0.322 Sodium chloride (8.6mg / ml) 0.307 Imported preparations 0.281
[0056] An isotonic solution has an osmolality of about 0.285-0.310 osmol / L.
Embodiment 3
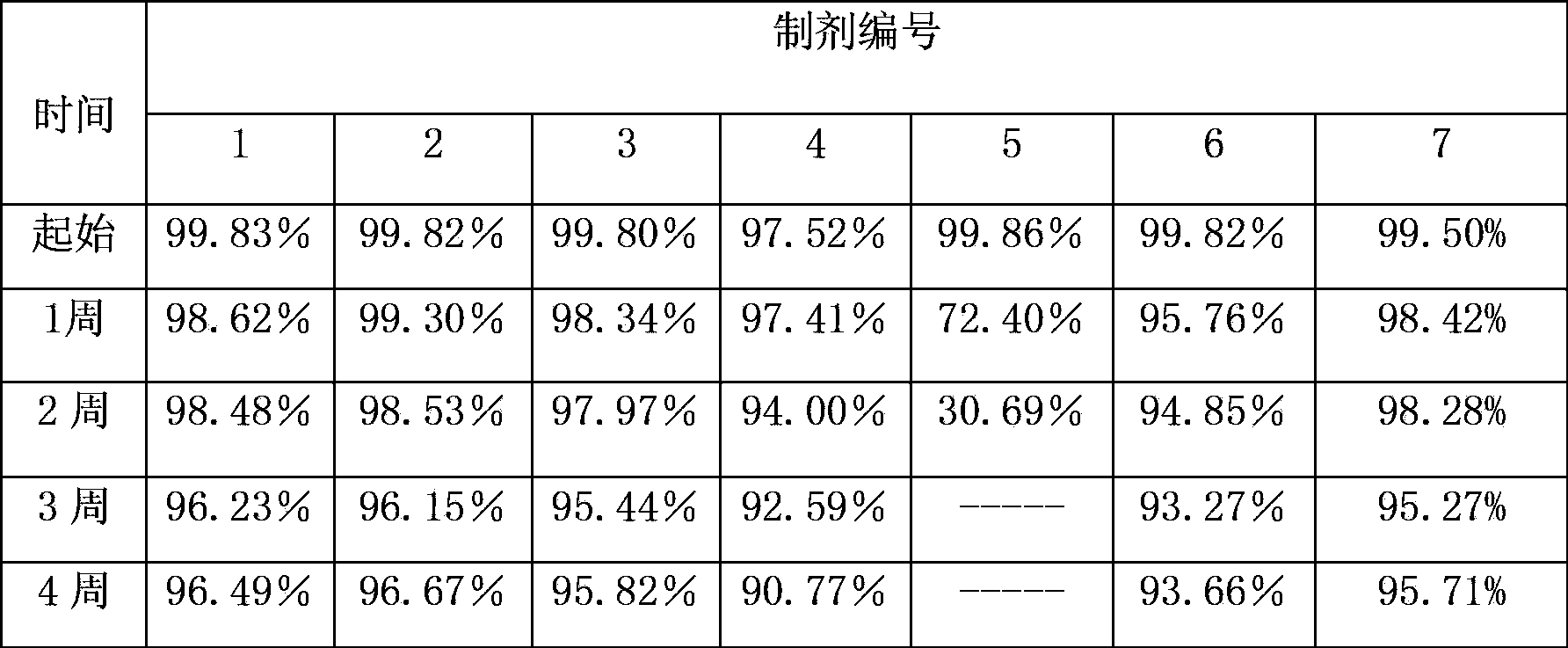
[0057] Example 3 Investigate the stability of formulation solutions containing different stabilizers
[0058] Dissolve preservatives, isotonic agents and buffers in water for injection, dissolve the original powder of liraglutide in the above solution while stirring slowly, and then adjust the pH to the required pH with sodium hydroxide and / or hydrochloric acid , respectively adding a certain amount of stabilizer. Finally, the above formulation solution was filtered with a 0.22 μm filter. The kind and consumption of adding stabilizer are shown in Table 3, and the composition of preparation is as follows:
[0059]
[0060] Table 3 Types and dosage of stabilizers
[0061] Preparation number Types of Stabilizers The amount of stabilizer 1 Polysorbate 80 0.02% 2 Poloxamer 188 0.02% 3 Polysorbate 80 + Poloxamer 188 0.01%+0.01% 4 Hydroxypropyl-β-cyclodextrin 2% 5 Povidone K30 3% 6 PEG300 3% 7 Listed prescription for ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap