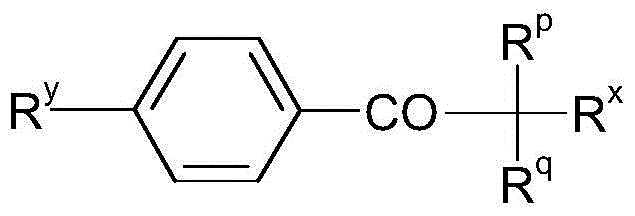
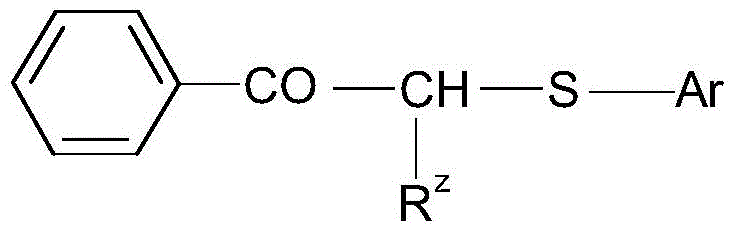
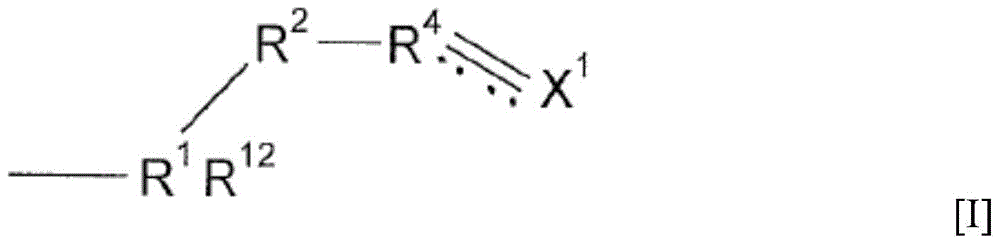Mineral processing using polymeric materials including moieties selectively bound to minerals
A polymeric material and polymer technology, applied in flotation, solid separation, etc., can solve the problem of collector chemical damage and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] Example 1 Attraction of copper sulfide, chalcopyrite, to the surface of a tetraenyl o-propyl quaternary ammonium polymer containing the collector chemical O,O-diethylphosphorothioate
[0149] method
[0150] Monomer N,N,N',N'-tetraallylpropane-1,3 - Dimethylammonium p-toluenesulfonate (>99%, 0.965g) and the synthesized monomers were dissolved in deionized water (0.080g) using gentle heating and vigorous mixing. Then, the photoinitiator "Irgacure2022" (Ciba SC) (0.0280 g) was added, followed by the collector chemical O,O-diethyl potassium thiophosphate (Sigma Aldrich (Sigma Aldrich, 90%, 0.0285g).
[0151] Small beads of this mixture were then placed on a PTFE plate and then a FusionUV LH6 high-intensity UV lamp with a D-bulb (bulb) was used using a single pass at a belt speed of 2 m / min. ) at 100% strength to solidify small beads of the mixture to produce a hard transparent solid.
[0152] The same materials were also used and samples were made without collector che...
Embodiment 2
[0161] Example 2 Attraction of copper sulfide, chalcopyrite, to tetraallyl o-propyl quaternary ammonium polymerization containing collector chemical O,O-diethylphosphorothioate after prolonged exposure to chalcopyrite the surface of the object
[0162] method
[0163] Experiment 1 was repeated except that the polymer beads with collector and reference samples without collector were placed in chalcopyrite and deionized water for 24 hours.
[0164] result
[0165] The collector-containing polymer beads were even darker in appearance compared to the collector-containing polymer beads that were left to stand for 4 hours. The difference in appearance between the beads with collector and the reference beads without collector was even greater than the difference in appearance after 4 hours.
Embodiment 3
[0166] Example 3 Use of Ultrasonic Treatment to Remove Copper Ore from the Surface of Tetraallylquaternary Ammonium Polymers
[0167] method
[0168] The monomer N,N,N',N'-tetraallylpropane-1,3-dimethylammonium p-toluenesulfonate (>99%, 1.47 g) was dissolved in deionized water ( 0.28g). The collector chemical O,O-diethyl potassium thiophosphate (SigmaAldrich, 90%, 0.13g) was dissolved in the mixture, followed by the addition of the photoinitiator "Irgacure2022" (Ciba SC) (about 40mg), and Simultaneously mix thoroughly.
[0169] A portion of the mixture was then placed between two glass slides and the mixture was mixed using two passes at 100% intensity at a belt speed of 4 m / min using a FusionUV LH6 high intensity UV lamp with a D-bulb lamp. Cured to yield a clear solid.
[0170] The polymer film was then recovered from the microscope slide and placed in a mixture containing approximately 200 mg of each of the following powders: Cu(I) sulfide (-325 mesh) in deionized water...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap