Preparation method of rivaroxaban
A technology of rivaroxaban and compounds, which is applied in the field of medicine, can solve the problems of long synthetic route, complex preparation process, and low total yield, and achieve the effects of mild reaction conditions, simple synthetic route, and high purity of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] A preparation method of rivaroxaban, comprising:
[0051] 1) 4-(4-iodophenyl)-3-morpholinone, (S)-(+)-N-(3-amino-2-hydroxyisopropyl)phthalimide and acid-binding Reagent reaction, obtain the compound with formula (I) structure,
[0052]
[0053] 2) converting the compound having the structure of formula (I) into rivaroxaban.
[0054]According to the present invention, the (S)-(+)-N-(3- Amino-2 hydroxyisopropyl) phthalimide, acid-binding agent mixed reaction, obtain the compound with formula (I) structure; Described acid-binding agent is preferably potassium carbonate, sodium carbonate, pyridine, triethylamine And one or more in DIPEA, more preferably salt of wormwood, sodium carbonate, pyridine, triethylamine or DIPEA, most preferably salt of wormwood; The solvent of described reaction is preferably tetrahydrofuran (THF), dioxane and DMF One or more, more preferably tetrahydrofuran, dioxane or DMF, most preferably tetrahydrofuran; the 4-(4-iodophenyl)-3-morpholinon...
Embodiment 1
[0082] The preparation of formula (I) compound
Embodiment 1-1
[0084] In a 5L three-necked flask, add 4-(4-iodophenyl)-3-morpholinone (30.3g, 100mmol), (S)-(+)-N-(3-amino-2-hydroxyisopropyl Base) phthalimide (24.2g, 110mmol), anhydrous potassium carbonate (13.8g, 100mmol), tetrahydrofuran solution (540ml, 10.0ml / g) were stirred, and the obtained suspension was gradually warmed to 64 ° C, refluxed , clarified, the product slowly precipitated after about 4 hours, kept the reflux reaction for 24 hours, allowed to cool to room temperature, filtered with suction, washed the filter cake with ether 200ml*3 times, sucked dry, and sent it to 45°C for 6 hours to obtain the formula (I) The compound of structure-g, the yield is 85.5%.
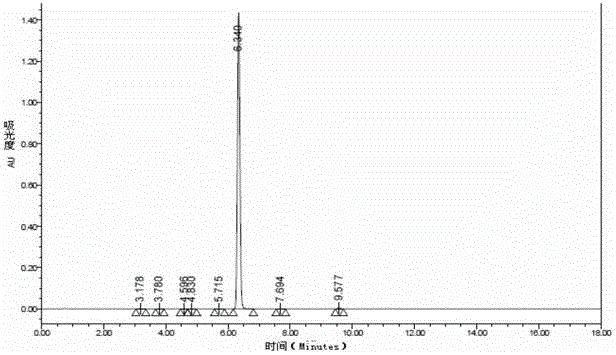
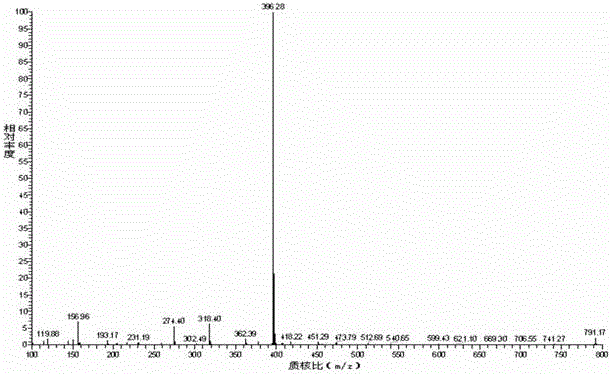
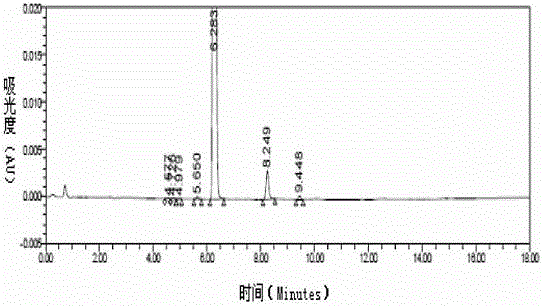
[0085] The compound of the formula (I) structure is detected by high performance liquid chromatography, and the results can be found in figure 1 , figure 1 It is the high-performance liquid chromatogram of the compound of the formula (I) structure described in Example 1-1, and the results show that its purity is 99....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap