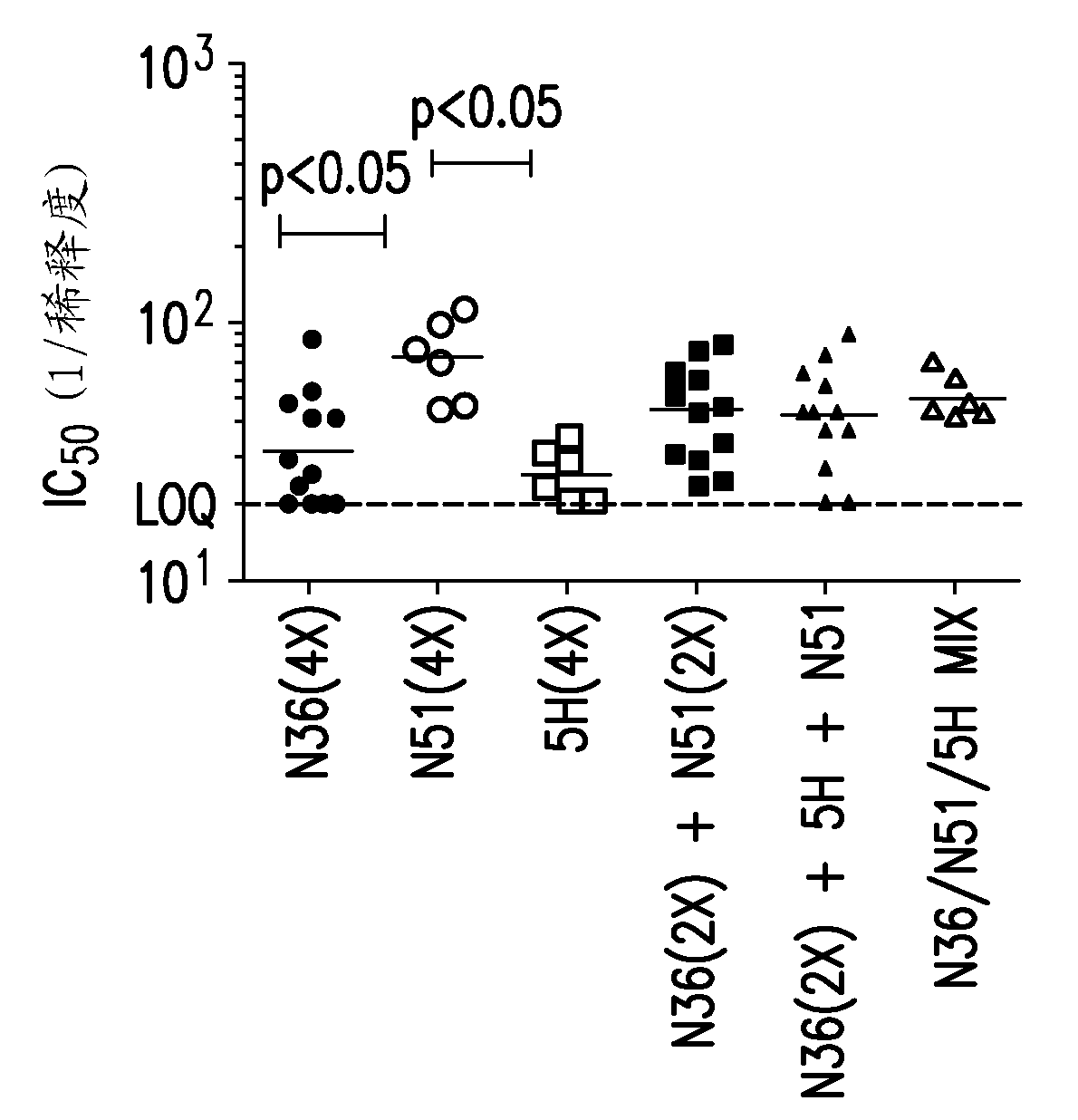Stable peptide mimetics of the HIV-1 GP41 pre-hairpin intermediate
A technology of peptidomimetic, bromide, applied in the field of trivalent gp41 peptidomimetic and its use as an immunogen to elicit neutralizing antibodies against HIV
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0177] Immunogen production and characterization
[0178] Immunogen Production: Synthetic Peptides
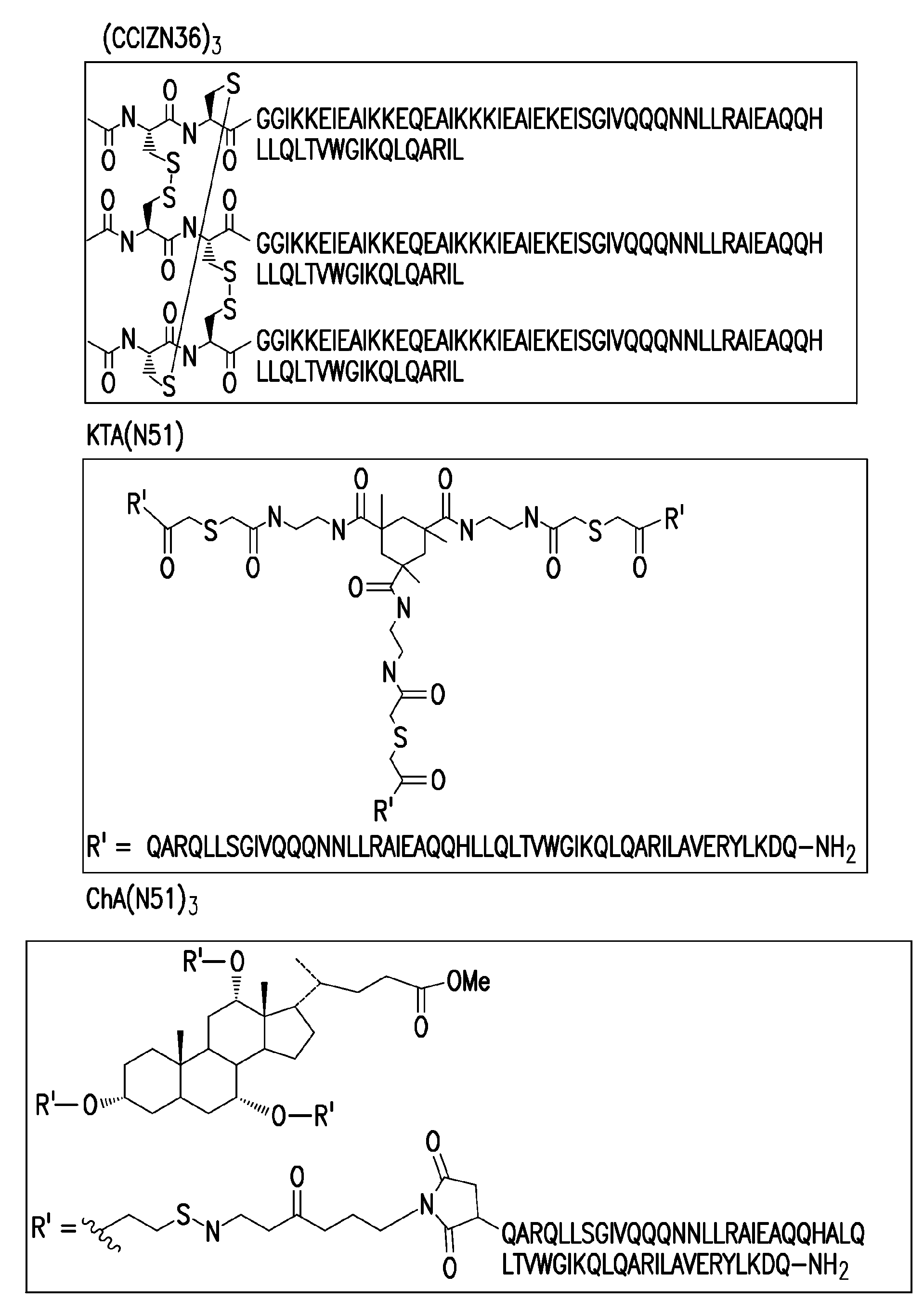
[0179] 1. (CCIZN36) 3
[0180] Peptide monomer CCIZN36
[0181] Synthesized on an automated peptide synthesizer using solid-phase Fmoc / t-Bu chemistry. The resin used was H-Rink Amide ChemMatrix (Matrix-Innovation Inc., St. Hubert, Quebec, Canada). Acylation was carried out using double coupling for 30 minutes, using a 5-10 fold excess of amino acid over the free amino groups of the resin per cycle. With an equimolar amount of HATU [2-(1H-9-azabenzotriazol-1-yl)-1,1,3,3-tetramethyl-urea (aminum) hexafluorophosphate] and 2 times A molar excess of DIEA (N,N-diisopropylethylamine) activates the amino acid. The side chain protecting groups used are as follows: trityl for cysteine, glutamine, asparagine, and histidine; tert-butoxycarbonyl for lysine and tryptophan; tert-butyl for for glutamic acid, threonine and serine; and 2,2,4,6,7-pentamethyldihydrobenzofuran-5-su...
Embodiment 2
[0254] Serology
[0255] 1. ELISA
[0256] Serum endpoint dilutions were determined by testing immune serum samples against biotinylated peptides added directly to streptavidin-coated 96-well plates (Thermo Fisher Scientific, Inc., Pittsburg, PA). Biotinylated peptides were coated at a concentration of 4 μg / ml / well in PBS overnight at 4°C. Plates were washed six times with PBS containing 0.05% Tween-20 (PBST) and blocked with PBST containing 3% (v / v) nonfat dry milk (PBST-milk). Test samples, pre-immune and immune samples were diluted, starting at 1:100, and serially diluted 4-fold, eight times in a final volume of 100 μl / well. Plates were incubated for 2 hours at room temperature, followed by six washes with PBST. Fifty microliters of HRP-conjugated goat anti-guinea pig (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) or goat anti-human (Invitrogen) secondary antibodies were diluted 1:5000 or 1:2000 in PBST-milk, respectively, and Add to each well and i...
Embodiment 3
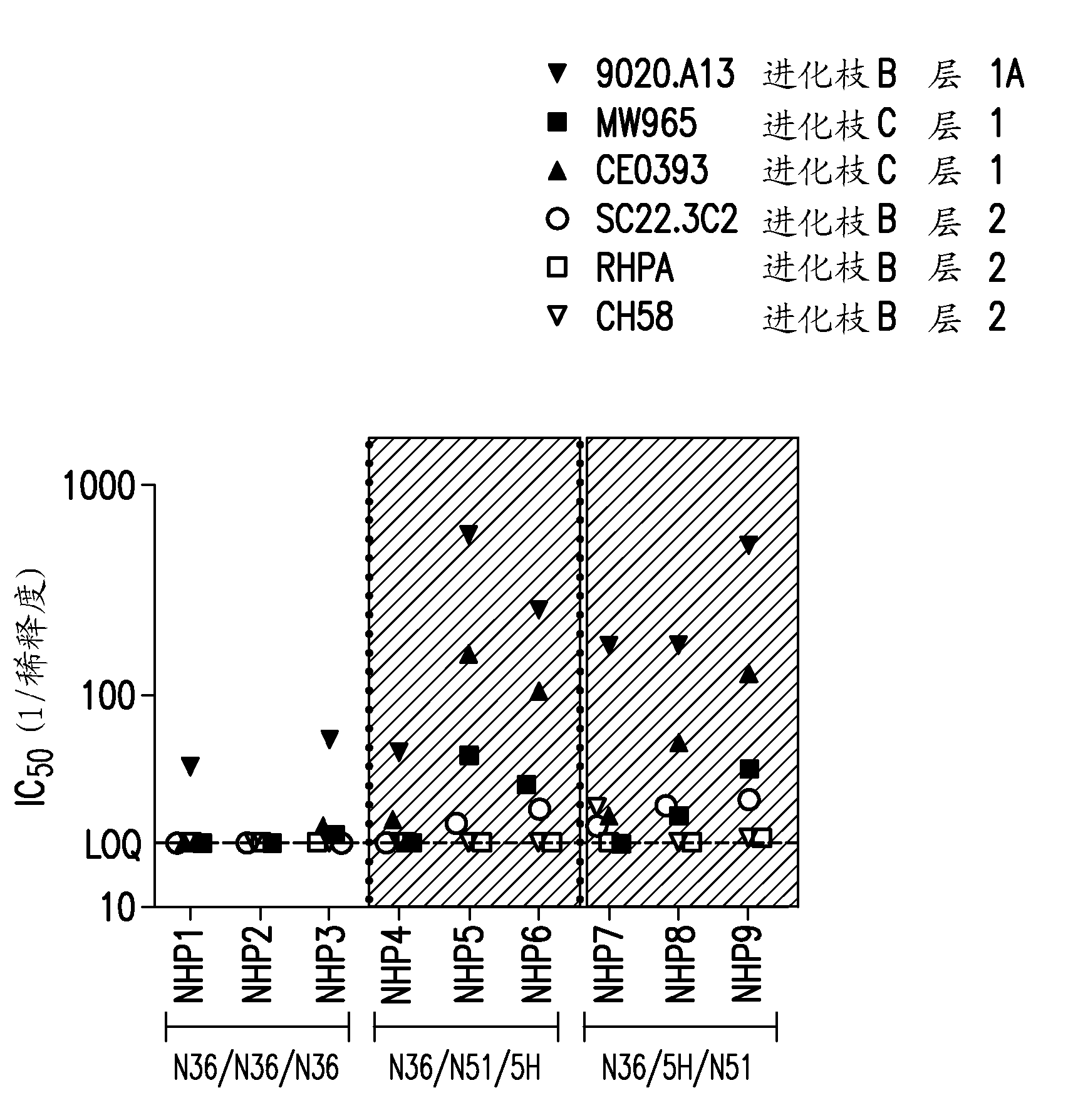
[0267] HIV 350 and 365: immunogenicity in guinea pigs
[0268] Duncan-Hartley guinea pigs (HIV-350, n=8 / group*) were immunized three times intramuscularly with 100 micrograms of the peptide immunogen at 0, 4 and 8 weeks. Peptides reconstituted at neutral pH in 20 mM Hepes buffer were formulated into 180 μg aluminum hydroxyphosphate sulfate (Merck & Co., Inc.) plus 40 μg Iscomatrix Adjuvant™ (CSL, Inc.) per dose. Serum samples were collected via whole blood in serum separator tubes for each animal at 7 and 1 week, as well as several serum collections prior to the first immunization (pre-bleed).
[0269] Studies of HIV-350 have tested the peptide construct SZN51. Table 3a The immunization schedule used in the study for this group is shown.
[0270] Duncan-Hartley guinea pigs (HIV-365, n=6 / group) were immunized three times intramuscularly with 30 μg of peptide immunogen at 0, 4 and 8 weeks. Peptides reconstituted at neutral pH in 20 mM HEPES buffer were formulated into 180 ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap