4‑Chloro‑2‑(5‑phenyl‑1‑(pyridine‑2‑yl)‑4,5‑dihydro‑1h‑pyrazole‑3‑yl)phenol in pharmaceutical applications
A technology of phenyl and pyridine, applied in 4-chloro-2-(5-phenyl-1-(pyridin-2-yl)-4,5-dihydro-1H-pyrazole-3-yl)phenol Fields of application in pharmaceuticals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Preparation of 4-chloro-2-(5-phenyl-1-(pyridin-2-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol (FPD5)
[0061] To a solution of chalcone (1.0 mmol) in ethanol (15 mL) was added 2-hydrazinopyridine (1.2 mmol) and sodium hydroxide (3.0 mmol). The reaction mixture was continued to reflux for 4 hours. The progress of the reaction was monitored by thin layer chromatography. After the reaction is complete, cool to room temperature, dilute with cold water, and neutralize with hydrochloric acid. After filtration, the resulting crude product was washed with water and ethanol, and recrystallized from ethanol. Yield 36.0%, mp: 172-173°C.
[0062] IR(KBr,cm -1 ):3066.0, 3031.5, 1588.9, 1476.0, 1443.9, 1255.9, 1145.2, 760.4, 692.9; 1 H NMR (400MHz, CDCl 3 ): δ3.28 (dd, 1H, J=5.6, 17.4Hz, 4-H trans ), 3.89 (dd, 1H, J=12.4, 17.4Hz, 4-H cis ), 5.81 (dd, 1H, J = 5.6, 12.4Hz, 5-H of pyrazoline), 6.70 (dd, 1H, J = 5.3, 6.7Hz, pyridine-H), 6.99 (d, 1H, J = 8.8Hz ,Ar-H),7.12(d,...
Embodiment 2
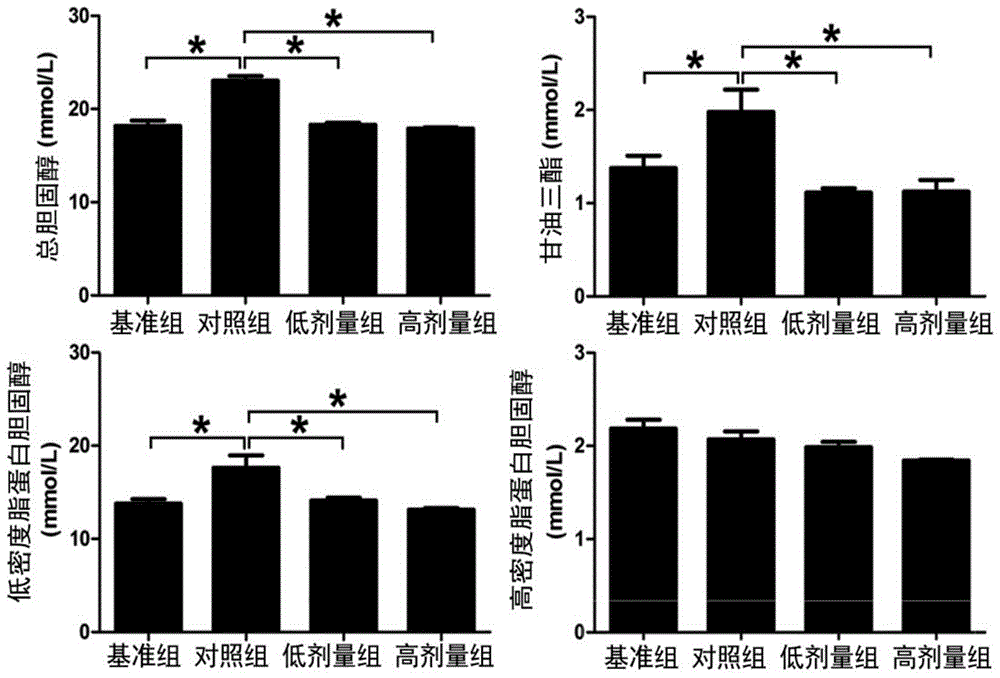
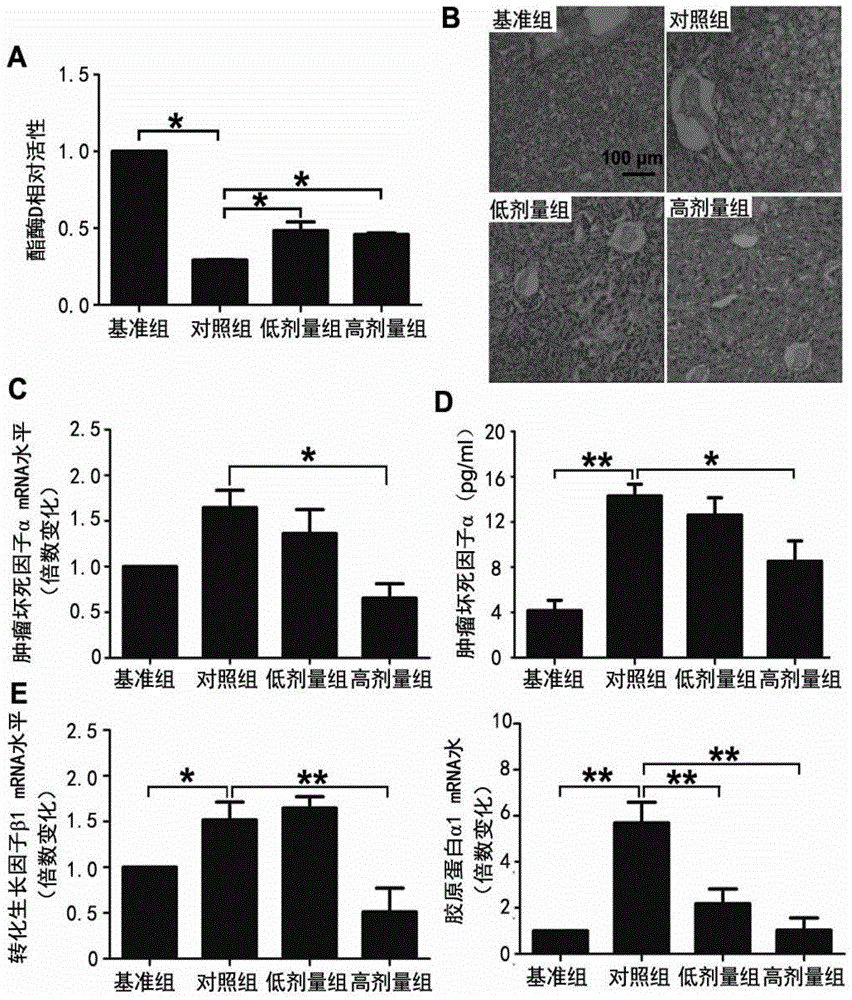
[0063] Example 2: FPD5 to apoE - / - Effects of Fatty Liver in Mice
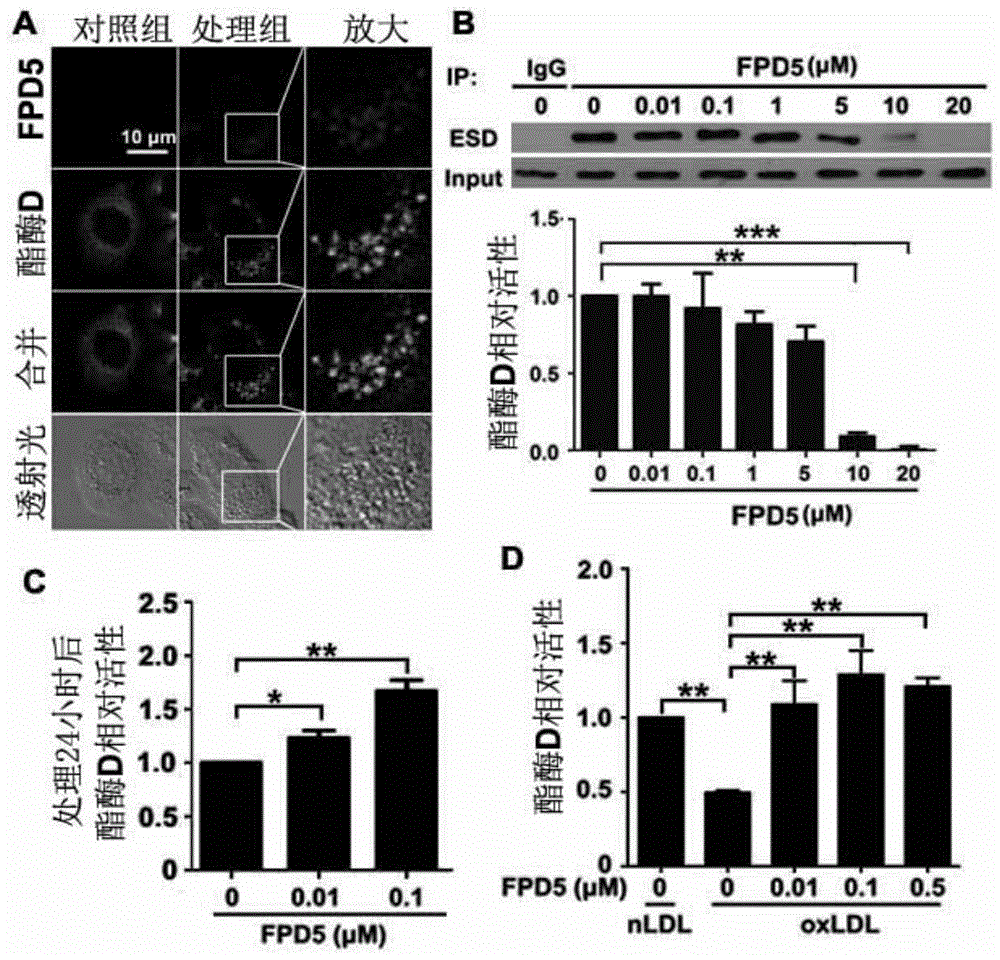
[0064] Based on previous experiments, it has been shown that FPD5 (0.01μM-0.5μM) can interact with esterase D (ESD), promote the activity of ESD, and at the same time, effectively inhibit the liver cells caused by oxidized low-density lipoprotein cholesterol (ox-LDL). Downregulation of ESD activity (see figure 1 ), the applicant chose doses of 5 μg / kg / day and 20 μg / kg / day to explore the effects of FPD5 on the stability of fatty liver and atherosclerotic plaque.
[0065] apoE - / - Mice (purchased by Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.) were fed with high-fat diet (formula: 0.25% cholesterol+15% lard+85% common feed) until the end of sampling.
[0066] Experimental mice were randomly divided into four groups: baseline group (no injection), control group (DMSO solvent / PBS: v / v=1:4, 100 μL), FPD5 high-dose group (20 μg / kg / day), FPD5 low dose group (5μg / kg / day).
[0067] The baseline ...
Embodiment 3
[0071] Example 3 FPD5 to apoE - / - Effects of Arterial Plaque Size in Mice
[0072] The aortic root isolated in Example 2 was frozen and embedded and sectioned for later use. The aorta was sectioned longitudinally, rinsed with PBS, and fixed in 10% neutral formalin for later use. The longitudinally sectioned aorta was stained with oil red O, and the sections were fixed with calcium formaldehyde for 10 minutes; washed three times with distilled water; dehydrated in 60% isopropanol for 1 minute; put in oil red O staining solution, and stained in a constant temperature box at 60°C for 20- 30min; decolorize the slices in 60% isopropanol for 1min; wash in distilled water for 1min; stain with hematoxylin for 30s; wash in distilled water for 1min; differentiate with 1% hydrochloric acid alcohol for 3s; The plaque area and vessel wall area were measured using ImagePro image analysis software, and the plaque / vessel wall area ratio was calculated. The frozen section of aortic root carr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com