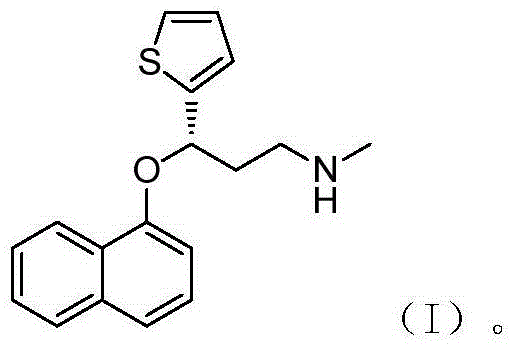
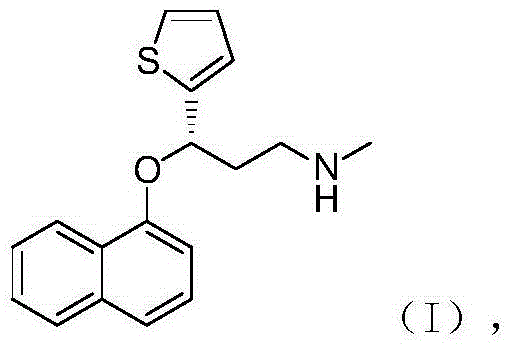
Method for preparing noradrenaline reuptake dual inhibitor
A methyl and xylene technology is applied in the field of preparation of dual serotonin and norepinephrine reuptake inhibitors, and can solve the problems of increased degradation by-products, inconvenient industrial production, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0045] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0046] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
[0047] In the present invention, mmol means millimole, h means hour, g means gram, ml means milliliter, and TBAB is tetrabutylammonium bromide.
Embodiment 1
[0048] The preparation of embodiment 1 duloxetine free base
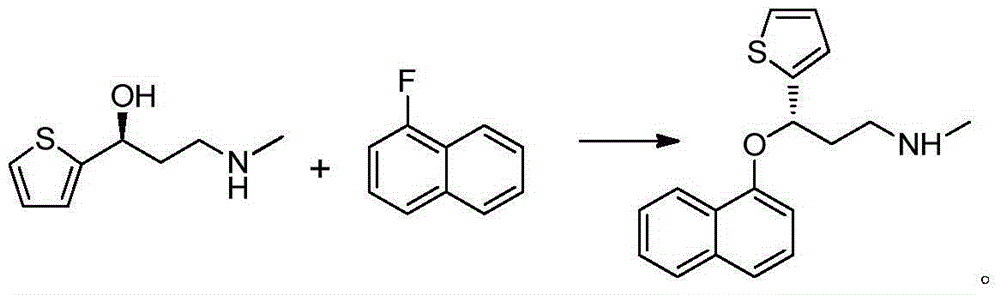
[0049] Add xylene (100mL), DMSO (10mL), powdered KOH (11.14g), (S)-(-)-N-methyl-3-hydroxyl-3-(2 -Thienyl)propylamine (10g), TBAB (1.13g) and 1-fluoronaphthalene (10.66g), heated to about 60 ° C, HPLC monitoring of the reaction process; after the reaction was completed, the reaction solution was cooled to 25-30 ° C, added The reaction was quenched with 100 mL of water, the organic phase was separated, washed with water (100 mL×2), and the organic phase was concentrated under reduced pressure to obtain 20 g of duloxetine free base, which was directly used in the next reaction.
Embodiment 2
[0050] The preparation of embodiment 2 duloxetine free base
[0051] Add xylene (100mL), DMSO (10mL), powdered KOH (11.14g), (S)-(-)-N-methyl-3-hydroxyl-3-(2 -Thienyl)propylamine (10g), TBAB (1.13g) and 1-fluoronaphthalene (10.66g), heated to about 80 ° C, HPLC monitoring of the reaction process; after the reaction was completed, the reaction solution was cooled to 25-30 ° C, added The reaction was quenched with 100 mL of water, the organic phase was separated, washed with water (100 mL×2), and the organic phase was concentrated under reduced pressure to obtain 21.5 g of duloxetine free base, 98.26% ee.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap