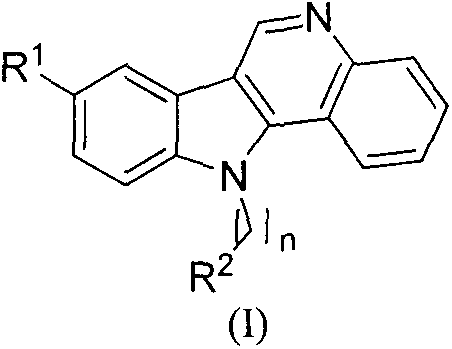
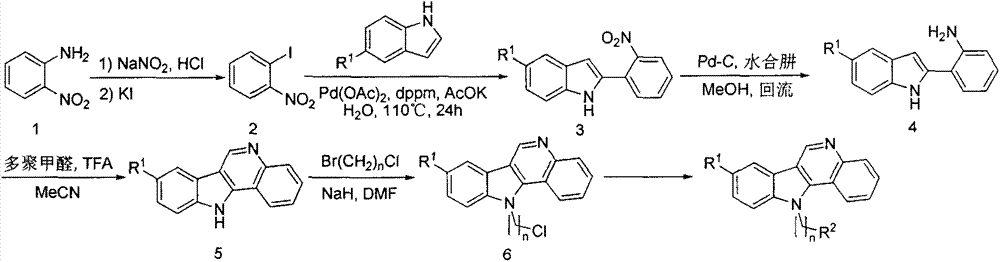
Preparation method and use of iso-cryptolepine derivatives
A technology of isolemenine and compound, which is applied to the preparation of 8-position, 11-position derivatives and 11-position derivatives of 5-desmethyl isolemenine, and the application field of preparing antitumor drugs, which can solve the problem of High cost, few studies on antitumor activity, weak antitumor activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The preparation method of 3-(11H-indo[3,2-c]quinolin-11-yl)propyl-1-amine
[0063] 1) Preparation of 2-nitroiodobenzene
[0064] Dissolve 1.38g (10mmol, 1equiv) of 2-nitroaniline in 5mL of concentrated hydrochloric acid, heat it to 100°C, keep it for 10 minutes to dissolve it, then cool it to 0°C with an ice-salt bath, add 0.83g ( 12mmol, 1.2equiv) Sodium nitrite was dissolved in 3mL of water, and slowly dropped into it under the condition of ice-salt bath. After dropping, the temperature of the reaction solution was raised to 70° C. for 2 to 3 hours. Cool the reaction solution to room temperature, add 15 mL of ethyl acetate to extract three times, combine the extracts, wash with dilute hydrochloric acid, sodium hydroxide solution, saturated sodium sulfite solution, and saturated sodium chloride solution in turn, dry with anhydrous sodium sulfate, and spin dry , and separated by column chromatography to obtain 2.82 g of a yellow solid, with a yield of 98.5%.
[0065]...
Embodiment 2
[0079] Preparation method of 4-(11H-indole[3,2-c]quinolin-11-yl)butyl-1-amine
[0080] Dissolve 170 mg (0.55 mmol, 1 equiv) of 11-(4-chlorobutyl) indole [3,2-c] quinoline in 5 mL of dimethyl sulfoxide, add 54 mg (0.83 mmol, 1.5 equiv) of sodium azide , reacted in an oil bath at 100°C for 12 hours. Cool the reaction solution, add 20 mL of water, extract with 60 mL of dichloromethane, wash the extract twice with water and saturated sodium chloride solution, dry over anhydrous sodium sulfate, and spin dry at low temperature to obtain 158 mg of oily azide. The rate is 91.1%.
[0081] Dissolve 158mg (0.5mmol, 1equiv) 11-(4-azidobutyl) indole [3,2-c] quinoline in a mixed solvent of 4.5mL tetrahydrofuran and 1.5mL water, add 262mg (1.0mmol, 2equiv ) triphenylphosphine, stirred at room temperature for 12 hours. The reaction solution was spin-dried, added 20 mL of water, extracted 3 times with 20 mL of ethyl acetate, combined the extracts, washed with saturated sodium chloride solut...
Embodiment 3
[0084] The preparation method of 11-(3-(1H-1,2,4-triazol-1-yl)propyl)-indole[3,2-c]quinoline
[0085] Dissolve 38 mg (0.55 mmol, 2 equiv) of 1,2,4-1H-triazole in 2 mL of dry DMF, add 13 mg (0.55 mmol, 2 equiv) of sodium hydrogen, pull out the hydrogen, stir at room temperature for 30 minutes, then dissolve 80 mg (0.27 mmol , 1equiv) 11-(3-chloropropyl)indole[3,2-c]quinoline was dissolved in 2mL DMF and dropped into the reaction solution, and reacted at room temperature for 3 hours. Add 20 mL of water to the reaction solution, extract three times with 20 mL of ethyl acetate, combine the extracts, wash with saturated sodium chloride solution, dry over anhydrous sodium sulfate, spin dry, and separate by column chromatography to obtain 32 mg of the product with a yield of 36.2%. .
[0086] Yellow solid, m.p.58-60℃; 1 H NMR (300MHz, CDCl 3 )δ9.38(s, 1H), 8.31(d, J=8.3Hz, 1H), 8.18(d, J=3.9Hz, 1H), 8.11-8.08(m, 2H), 7.71(t, J=7.4 Hz, 1H), 7.60-7.53(m, 2H), 7.48-7.40(m, 3H), 4.87...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com