Application of (E)-N-piperonyl-1-(3-(6-chloropyridine)methyl)-3-phenyl-1H-pyrazole-5-carbohydrazide in medicament pharmacy
A kind of chloropyridine, piperonyl technology, applied in (E)-N-piperonyl-1-(3-(6-chloropyridine)methyl)-3-phenyl-1H-pyrazole-5-carbonyl The application field of hydrazine in pharmaceuticals has the effect of inhibiting the formation of microtubules and having good development and application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0034] Example 1:
[0035] (E)-N-piperonyl-1-(3-(6-chloropyridine)methyl)-3-phenyl-1H-pyrazole-5-carbohydrazide
[0036] 1) Add potassium carbonate (0.005 mol), 3-phenyl-1H-pyrazole-5-ethyl carboxylate (0.005 mol), 2-chloro-5-chloromethylpyridine (0.005 mol) to a 100 ml round bottom flask Mol) and acetonitrile (25 ml), the device reflux condenser, the upper part is connected to the drying tube. Heat to reflux for 4 hours, react until the raw materials are completely consumed, and detect the end of the reaction by TLC. Concentrate under reduced pressure, remove the solvent, add ethyl acetate (30 ml), filter, and concentrate the filtrate. Use ethyl acetate-petroleum ether (V / V=1 / 2) as the eluent to separate the residue (100~ 200 mesh silica gel) to obtain 1-(3-(6-chloropyridine)methyl)-3-phenylpyrazole-5-carboxylic acid ethyl ester with a yield of 87%.
[0037] 2) To 0.341 g (0.001 mole) of 1-(3-(6-chloropyridine)methyl)-3-phenylpyrazole-5-carboxylic acid ethyl ester in methanol (5 ...
Example Embodiment
[0051] Example 2:
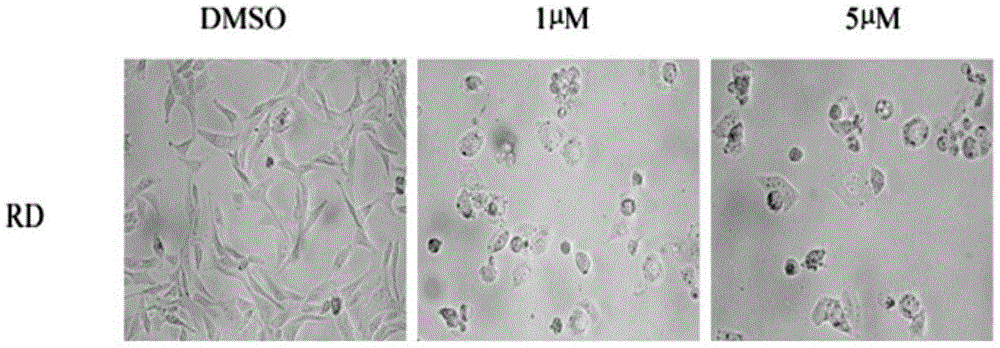
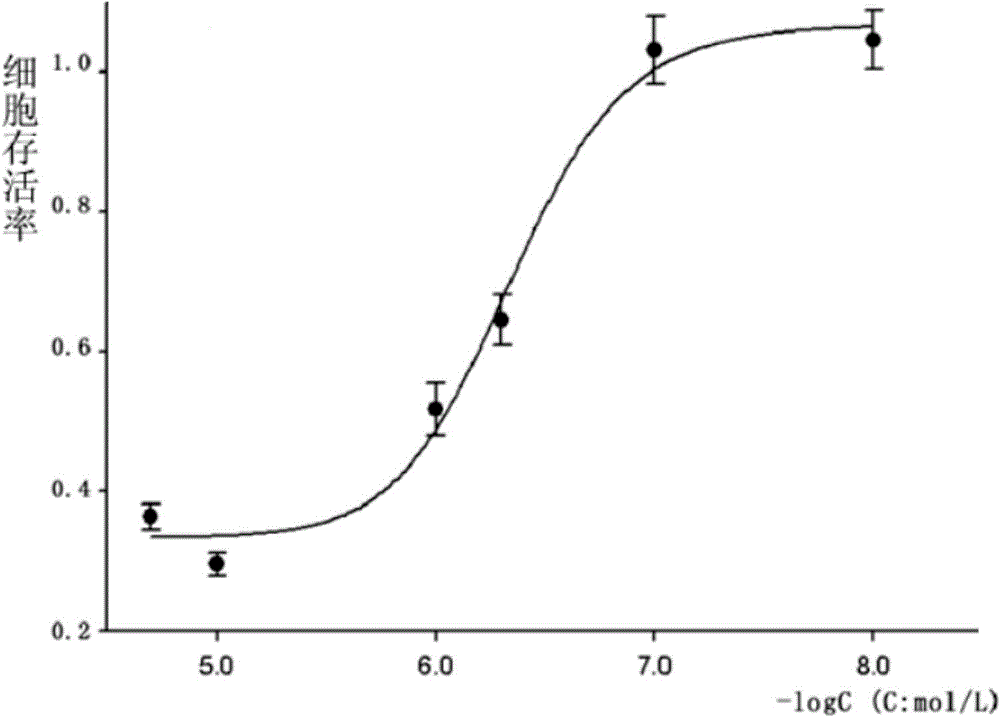
[0052] Determination of (E)-N-piperonyl-1-(3-(6-chloropyridine)methyl)-3-phenyl-1H-pyrazole-5-carbohydrazide on the half-inhibitory concentration of rhabdomyosarcoma cell growth by SRB method
[0053] At 37°C and 5% CO 2 Under the environment, human rhabdomyosarcoma RD cells were cultured with DMEM / 10% fetal bovine serum medium. Collect logarithmic phase human rhabdomyosarcoma RD cells, inoculate them in 96-well plates, and incubate for 24h. Add (E)-N-piperonyl-1-(3-(6-chloropyridine)methyl)-3-phenyl-1H at different concentrations (20, 10, 5, 1, 0.1, 0.05, 0.01μM) -Pyrazole-5-carbohydrazide was incubated for 48h, 50μL of trichloroacetic acid solution (30%) was added and fixed at 4°C for one hour. Shake off the solution, rinse with high-purity water five times, add 100 μL of sulforhodamine B to stain for 30 minutes after drying, and rinse with 1% acetic acid solution five times after spin-drying. After drying, add 100μL of Tris solution to fully dissolve, and ...
Example Embodiment
[0055] Example 3:
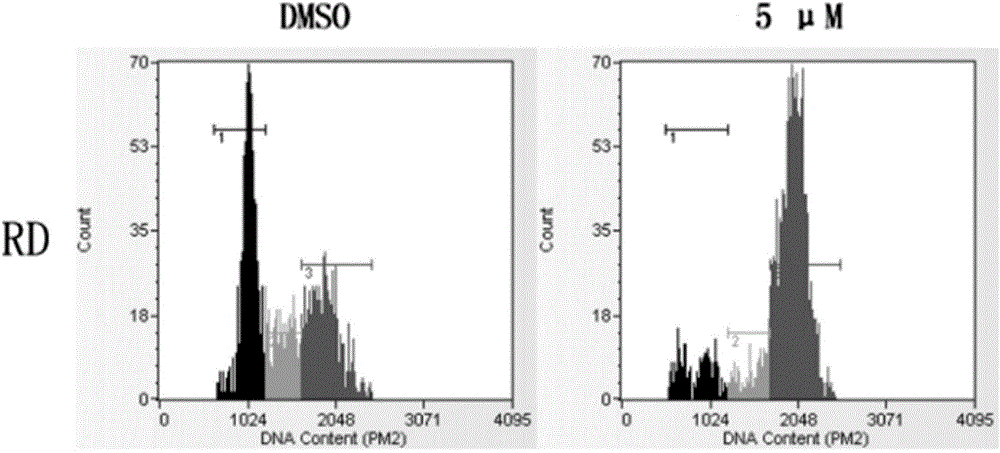
[0056] PI staining flow cytometry determination of (E)-N-piperonyl-1-(3-(6-chloropyridine)methyl)-3-phenyl-1H-pyrazole-5-carbohydrazide on rhabdomyosarcoma cells Cycle impact
[0057] Human rhabdomyosarcoma RD cells in the logarithmic growth phase were inoculated into 6 cm petri dishes at an amount of 200,000 cells per 3 mL of medium volume, and a total of 8 petri dishes were inoculated. After 24 hours, the 8 petri dishes were equally divided into two groups. DMSO was added to two petri dishes of each group, and 5.0 μM compound (E)-N-piperonyl-1-(3-(6-chloropyridine)methyl)-3-phenyl- was added to two petri dishes. 1H-pyrazole-5-carbohydrazide. After continuously incubating for 24 hours, the cells were digested on ice, the cell suspension was collected, centrifuged and washed twice with PBS. Add 10 ml of 70% ice ethanol and suspend it at -20°C overnight. The cell suspension was centrifuged, washed twice with PBS, 500μL PI / 5μL RNAaseA was added to the cells a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com