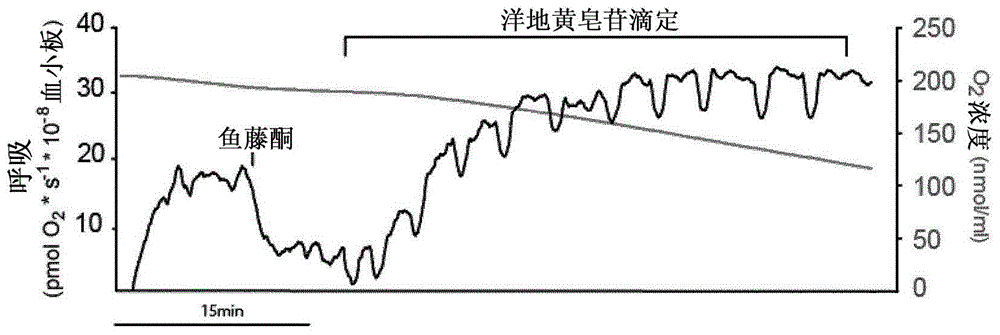
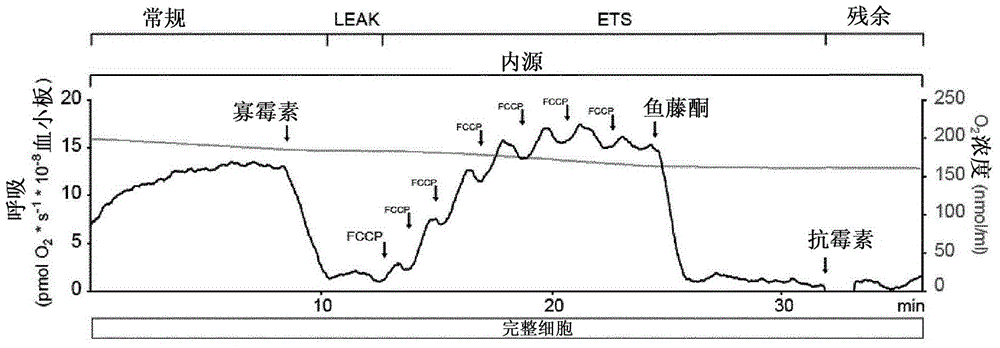
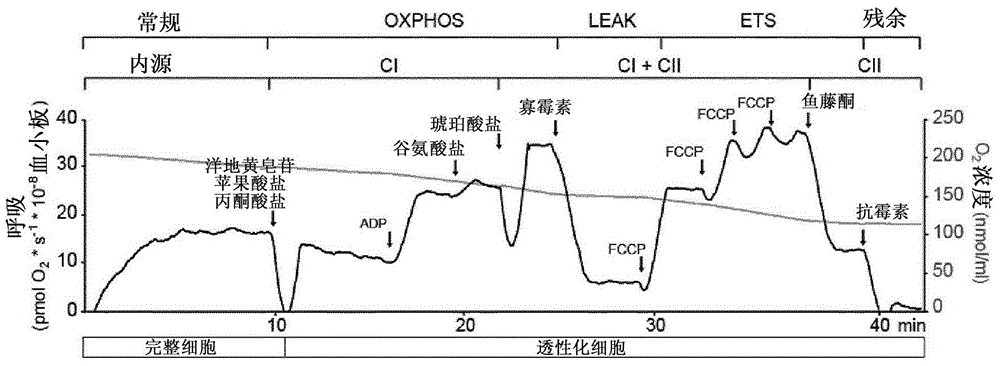
Mitochondrial toxicity test
A technology of mitochondria and mitochondrial complexes, applied in the detection of programmed cell death, biological testing, measurement devices, etc., can solve problems that are difficult to translate into human applications, and achieve the effect of reducing time and cost and avoiding pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0150] Experimental part
[0151] Materials and methods
[0152] 1.1 Obtaining human samples
[0153] This study was approved by the regional ethical review board of the Rand region in Sweden (adults: 113 / 2008 and 644 / 2009, children: 59 / 2009) and the ethics committee of Tokyo Medical University in Japan (permission No. 1514) approval. For adults in Sweden, from Landskåne University Hospital ( The Blood Donation Center at the University Hospital collects blood samples from healthy donors, as well as from healthy adults recovering from knee injuries. The Japanese group consisted of healthy adult volunteers. Samples were obtained after obtaining written informed consent. Trials were conducted according to the same procedures and protocols and by the same investigators at both research stations. Pediatric control samples were obtained from patients undergoing minor elective surgery. Written informed consent was obtained from parents or guardians, and blood was drawn before...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com