A kind of phenanthroimidazole derivative and application thereof
A technology of phenanthroimidazole and derivatives, which is applied to phenanthroimidazole derivatives and their application fields, can solve the problems of low mobility, difficult to prepare high-efficiency OLED devices, and needs to be improved, and achieves improved electron transport ability, Good electron/hole transport ability, the effect of improving external quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
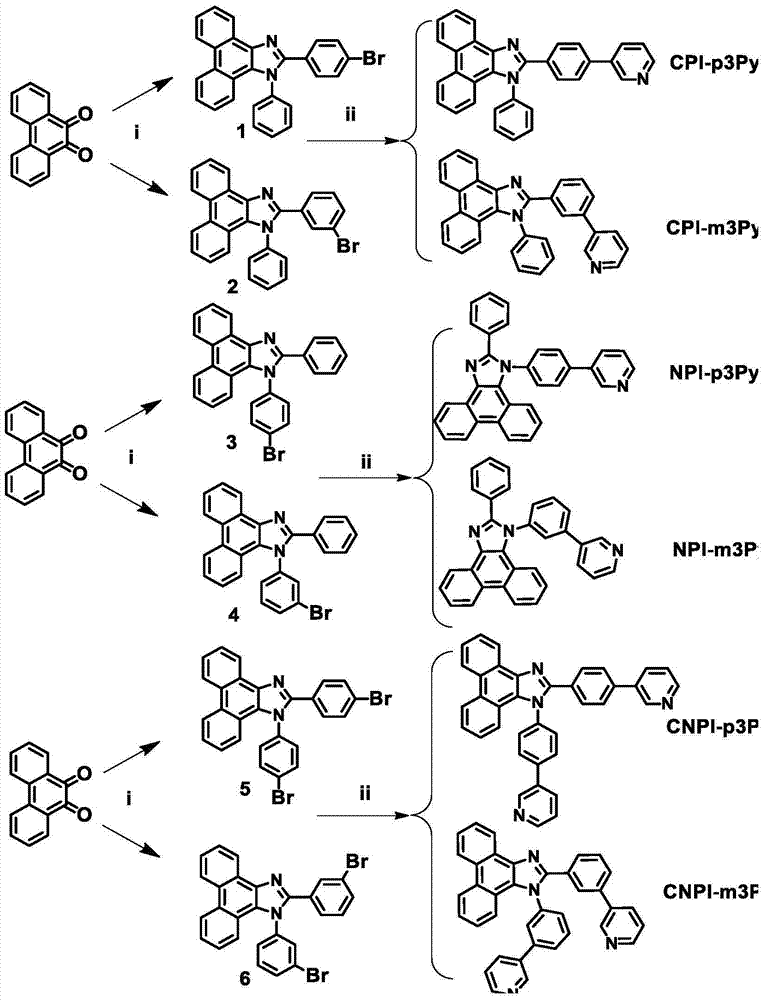
[0038] A kind of phenanthroimidazole derivative, it has the structure of formula (I), wherein R 1 for pyridine ie R 2 is a hydrogen atom, R 3 is a hydrogen atom, R 4 It is a hydrogen atom; its chemical name is 2-(4-(3-pyridine)phenyl)-1-phenyl-1H-[9,10-d]phenanthroimidazole, referred to as CPI-p3Py, and its structural formula is as follows:
[0039]
[0040] Its preparation method is as follows: under the protection of nitrogen, compound 2-(4-bromophenyl)-1-phenyl-1H-[9,10-d]phenanthroimidazole (1) (1.76g, 4.0mmol) , 3-pyridine boronic acid (0.50g, 4.0mmol), toluene (100ml), absolute ethanol (50ml), 2M K 2 CO 3 (50ml) aqueous solution was successively added to a dry 500ml two-necked round-bottomed flask, ultrasonicated for 30 minutes, under nitrogen conditions, the reaction was heated to 80°C, stirred and refluxed for 24 hours, and the reaction was cooled to room temperature, and dichloromethane (60ml) was added. Extracted three times, collected the organic phase and...
Embodiment 2
[0042] A kind of phenanthroimidazole derivative, it has the structure of formula (I), wherein R 1 for pyridine ie R 2 is a hydrogen atom, R 3 is a hydrogen atom, R 4 It is a hydrogen atom; its chemical name is 2-(3-(3-pyridine)phenyl)-1-phenyl-1H-[9,10-d]phenanthroimidazole, referred to as CPI-m3Py, and its structural formula is as follows:
[0043]
[0044] The synthesis process is the same as that of the compound CPI-p3Py to obtain 1.50 g of white solid 2-(3-(3-pyridine)phenyl)-1-phenyl-1H-[9,10-d]phenanthroimidazole (CPI-m3Py) . Yield: 84%. 1 H-NMR (CDCl 3 ,400MHz): δ(ppm)8.933~8.913(d,1H),8.799~8.778(d,1H),8.738~8.717(d,1H),8.628~8.611(d,2H),7.777~7.660(m, 8H), 7.599~8.534 (m, 4H), 7.463~7.434 (t, 1H), 7.296~7.235 (m, 3H). 13 C-NMR (CDCl 3 ,400MHz):δ(ppm)150.19,148.61,148.18,138.82,137.64,137.44,134.37,131.29,130.36,129.98,129.37,129.20,129.13,128.33,128.27,128.04,127.43,127.36,127.16,126.35,125.74, 125.05, 124.16, 123.57, 123.16, 122.97, 122.76, 120.91. MS(...
Embodiment 3
[0046] A kind of phenanthroimidazole derivative, it has the structure of formula (I), wherein R 1 is a hydrogen atom, R 2 for pyridine ie R 3 is a hydrogen atom, R 4 It is a hydrogen atom; its chemical name is 2-(4-(3-pyridine)phenyl)-1-phenyl-1H-[9,10-d]phenanthroimidazole, referred to as NPI-p3Py, and its structural formula is as follows:
[0047]
[0048] The synthesis process was the same as that of the compound CPI-p3Py, and 1.46g of white solid 2-(4-(3-pyridine)phenyl)-1-phenyl-1H-[9,10-d]phenanthroimidazole (NPI-p3Py) was obtained . Yield: 82%. 1 H-NMR (CDCl 3 ,400MHz): δ(ppm)9.031~9.025(d,1H),8.939~8.920(d,1H),8.813~8.792(d,1H),8.746~8.704(t,2H),8.045~8.025(d, 1H), 7.838~7.761(m,3H), 7.689~7.619(m,5H), 7.557~7.531(m,1H), 7.489~7.458(m,1H), 7.360~7.284(m,5H). 13 C-NMR (CDCl 3 ,400MHz):δ(ppm)151.03,149.28,148.31,139.10,138.56,137.42,135.04,134.51,130.31,129.83,129.54,129.34,129.01,128.58,128.34,128.31,128.02,127.38,127.11,126.37,126.37, 125.76, 125.02, 124....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com