Novel compound and organic electroluminescent device including the same
A technology of organic light-emitting devices and compounds, applied in the field of novel compounds and organic light-emitting devices containing them, can solve problems such as short life, high driving voltage, and low efficiency, and achieve the effects of preventing recrystallization, high color purity, and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
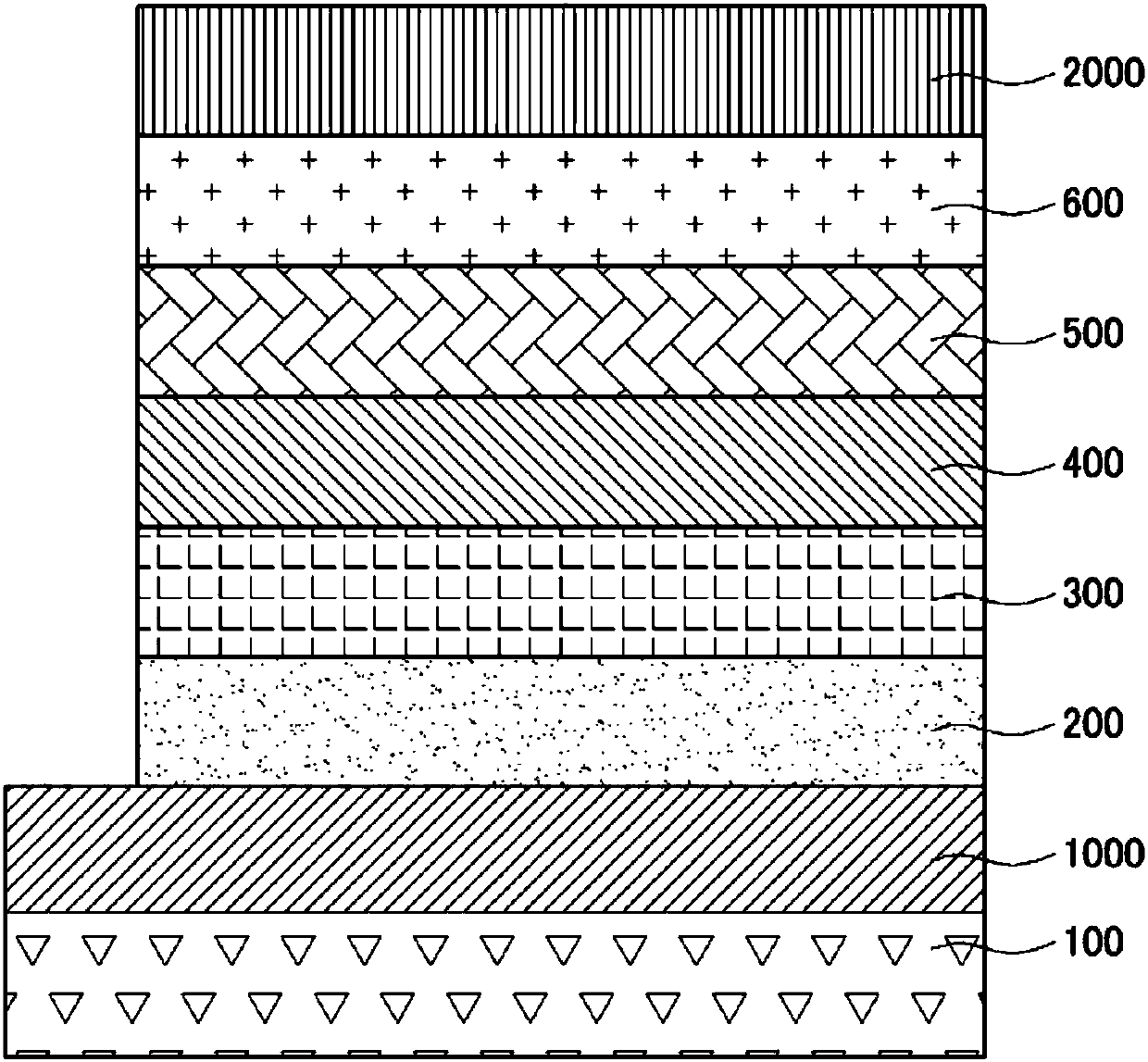
Image
Examples
no. 1 approach
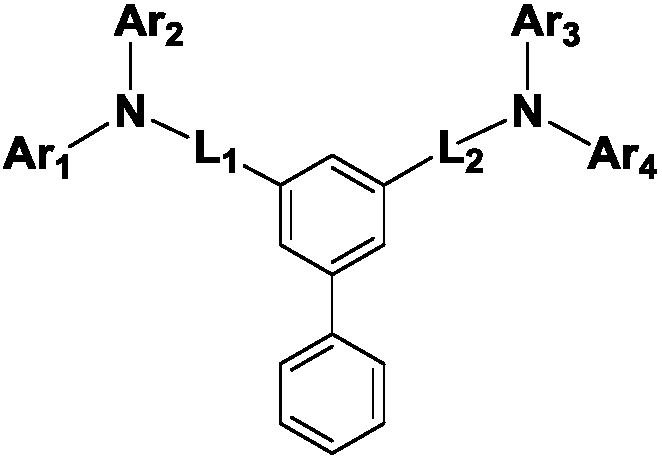
[0035] The first embodiment of the present invention provides a compound represented by the following chemical formula 1:
[0036] chemical formula 1
[0037]
[0038] In Chemical Formula 1,
[0039] Ar 1 for
[0040] Ar 2 to Ar 4 independently represent phenyl, biphenyl or naphthyl,
[0041] L 1 and L 2 Each independently represents straight chain, or substituted or unsubstituted C 5 -C 30 Arylene,
[0042] In the above Ar 1 In, * means the connection site.
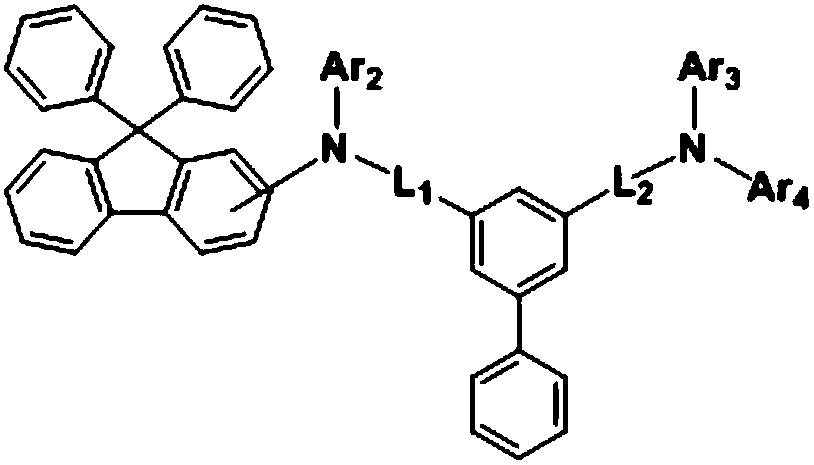
[0043] Ar above 1 It is one of diphenylfluorene, spirobifluorene, triphenylene, dibenzofuran or dibenzothiophene, thereby forming a HOMO with easy hole injection and transport, forming a low driving voltage, and achieving high efficiency and long-term long-life organic light-emitting devices.
[0044] In an example of the present invention, the compound of the above-mentioned Chemical Formula 1 may include a compound represented by the following Chemical Formula 2-1, Chemical Formula 2-2 or Chemical ...
preparation example 1
[0108] Preparation Example 1: Synthesis of Intermediate I-1
[0109]
[0110] Under argon or nitrogen atmosphere, in diphenylamine 8.46g (50mM), 3,5-dibromo-1,1'-biphenyl 21.84g (70mM), Pd 2 (dba) 3 1.6g(1.7mM), 50%P(t-Bu) 3 Add 300 ml of toluene to 2 ml (4 mM) and 14.7 g (152 mM) of NaOtBu, reflux for 7 hours, and heat. After the reaction, filter immediately, extract with dichloromethane, add MgSO 4 ,filter. After removing the solvent of the filtered organic layer, purification was performed by column chromatography, whereby intermediate I-1 (5-bromo-N,N-diphenyl-[1,1'-biphenyl]-3-amine was obtained ) 16.41 g. (Yield: 82%)
[0111] m / z: 399.06 (100.0%), 401.06 (97.1%), 402.06 (25.4%), 400.07 (16.3%), 400.07 (9.5%), 403.07 (1.8%), 401.07 (1.6%), 403.07 (1.5%) ), 401.07 (1.4%)
preparation example 2
[0112] Preparation Example 2: Synthesis of Intermediate I-2
[0113]
[0114] Except that 12.27 g of N-phenyl-[1,1'-biphenyl]-4-amine was used instead of diphenylamine, the same reaction as in Preparation Example 1 was carried out to obtain Intermediate I-2 ( N-([1,1'-biphenyl]-4-yl)-5-bromo-N-phenyl-[1,1'-biphenyl]-3-amine) 19.54 g. (Yield: 82%)
[0115] m / z: 475.09 (100.0%), 477.09 (97.1%), 478.09 (30.4%), 476.10 (17.4%), 476.10 (16.1%), 477.10 (4.5%), 479.10 (3.6%), 477.10 (1.3%) ), 479.10 (1.2%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com