Peptides and their uses
An integer, free technology, applied in the field of molecular biology and biochemistry, can solve the problems of the increase of antibiotic-resistant bacteria and the overuse of antibiotics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
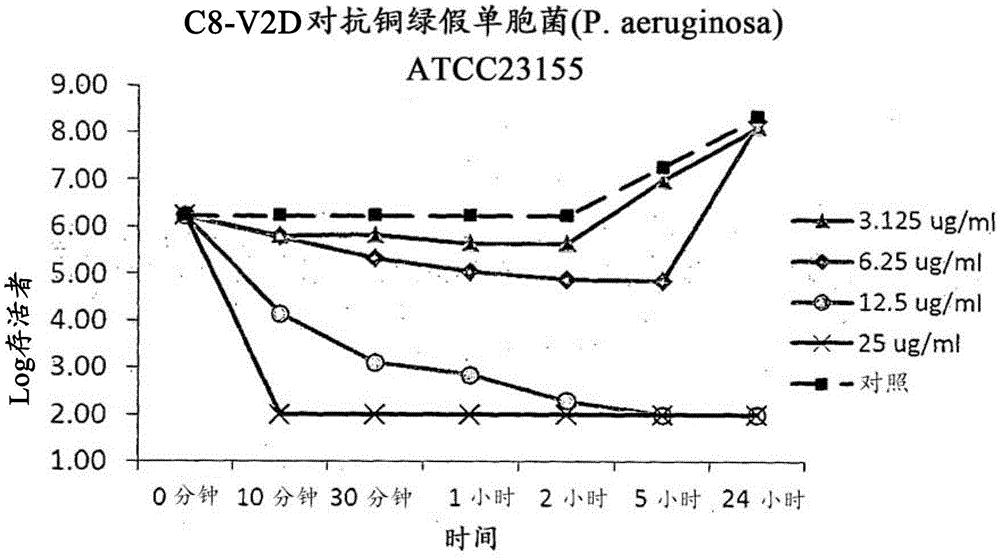
[0171] Example 3 Time-kill kinetics
[0172] Materials and Methods
[0173] The bacteria used in the time-kill study were isolated from 18-20 hour Tryptic Soy Agar (TSA) plates. Then, the inoculum was suspended in 0.31 mM phosphate buffer and adjusted to obtain 10 5 To 10 6 CFU / mL bacterial suspension. Then, the inoculum was treated with various concentrations of C8V2D. The mixture was incubated at 35°C. Remove aliquots of the culture at 0 minutes, 10 minutes, 30 minutes, 1 hour, 2 hours, 5 hours, and 24 hours for the plate viable count. Use Dey-Engley Neutralization Broth (Dey-Engley Neutralization Broth) 10-fold serial dilution aliquots, and then use the surface coating plate method to spread 20 μL of each dilution onto TSA flat. Then, the plate was incubated at 35°C for 48 hours to 72 hours. Cell viability was evaluated by counting the colonies grown on the plate. Bactericidal activity was defined as a 3 log reduction in viable counts (3-log) in cultures treated with anti...
Embodiment 4
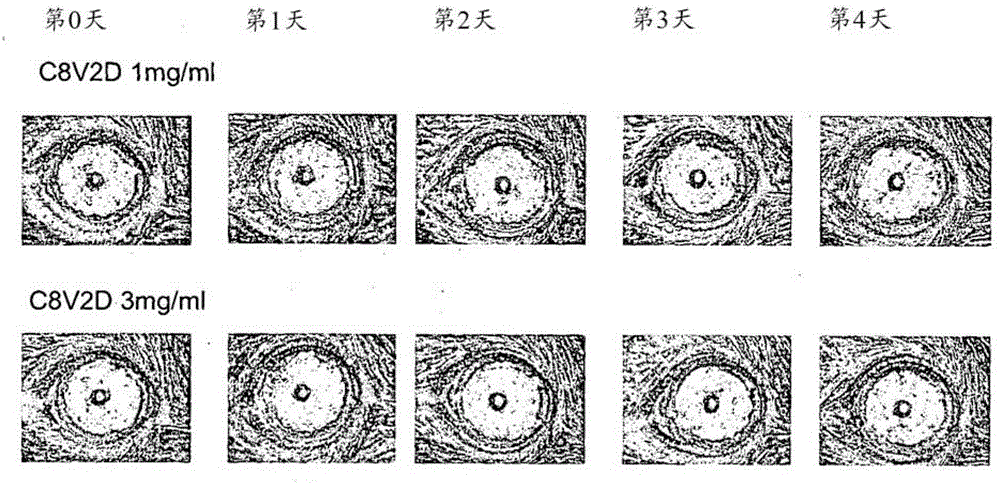
[0175] Example 4 In vitro and in vivo toxicity
[0176] 4A. Determination of hemolysis
[0177] Materials and Method
[0178] Fresh red blood cells (RBC) from New Zealand white rabbits were used in this experiment. The obtained RBC was centrifuged at 3000 rpm for 10 minutes. The supernatant was removed and washed twice with sterile PBS buffer (20mM, 100mM NaCl, pH 7). The RBC was further diluted to 8% stock solution in PBS. The peptide is dissolved in PBS at the desired storage concentration. When the compound was mixed with RBC, the desired concentration of 4% RBC was obtained. For C14V2D and C16V2D, dissolve them in dimethylformamide ((CH 3 ) 2 NCH; DMF). When the compound was mixed with RBC, the desired concentration of 4% RBC and 0.5% DMF was obtained. A DMF concentration of less than 1% was used because it has negligible hemolytic activity on RBC. The mixture was added to a 1.5 mL centrifuge tube and incubated at 37°C for 1 hour. After incubation, the blood was centrifu...
Embodiment 5
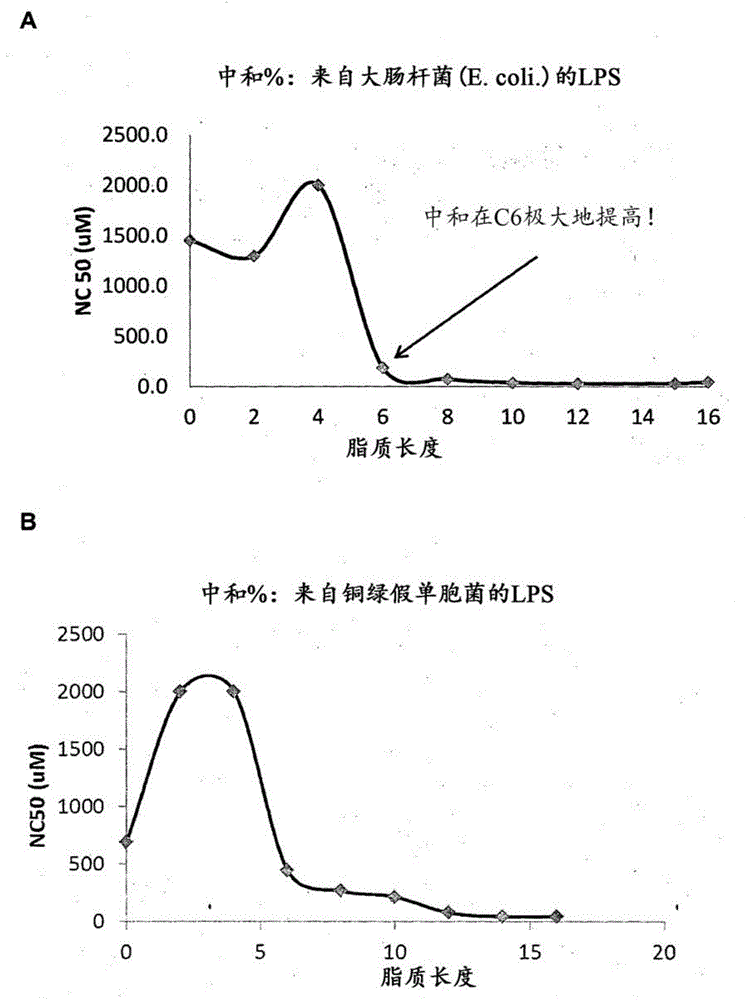
[0206] Example 5 Lipopolysaccharide (LPS) neutralization study
[0207] Study the possible effects of peptides and lipid-modified peptides of the present disclosure on LPS of Gram-negative bacteria
[0208] Materials and Method
[0209] The lipopolysaccharide (LPS) neutralization was evaluated by using the Pierce Limulus Amebocyte Lysate (LAL) Chromogenic Endotoxin Quantitative Kit (Thermo Scientific) with minor modifications to the manufacturer's protocol. LAL contains enzymes that are activated in a series of reactions in the presence of LPS. The last enzyme activated in the cascade splits the chromophore from the chromonic substrate into p-nitroaniline (PNA) and produces a yellow color. The amount of PNA released will be measured photometrically at 405 nm, which is proportional to the amount of LPS in the system. In short, first equilibrate the microplate in a heating module at 37°C for 10 minutes. Then, the lipid-modified peptide of the desired concentration was pipetted into...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap