Application of NADPH in preparation of drugs used for treating heart diseases
A technology for heart disease and medicine, which is applied in the application field of NADPH in the preparation of medicine for treating heart disease, and can solve the problems of reducing pentose metabolic activity, aggravating myocardial injury, increasing glycolysis and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 contains the capsule of NADPH
[0021] The capsule of the present embodiment comprises following composition:
[0022] NADPH 20g; suspending agent microcrystalline cellulose 60g; preservative tert-butyl-4-hydroxyanisole 0.04g; lubricant magnesium stearate 2g; filler lactose added to 200g.
[0023] Its preparation method comprises the following steps:
[0024] Weigh the NADPH and various pharmaceutical excipients in the above prescription amount, mix them evenly, pass through a 60-mesh sieve three times, and put them into capsules.
[0025] The conventional excipients used in the medicine include but are not limited to one or a mixture of fillers, disintegrants, lubricants, binders, flavoring agents, suspending agents, and preservatives.
[0026] Specifically, the filler can also be replaced with a mixture of one or more of pregelatinized starch, mannitol, chitin, microcrystalline cellulose, and sucrose;
[0027] The disintegrant can also be replaced by o...
experiment example 1
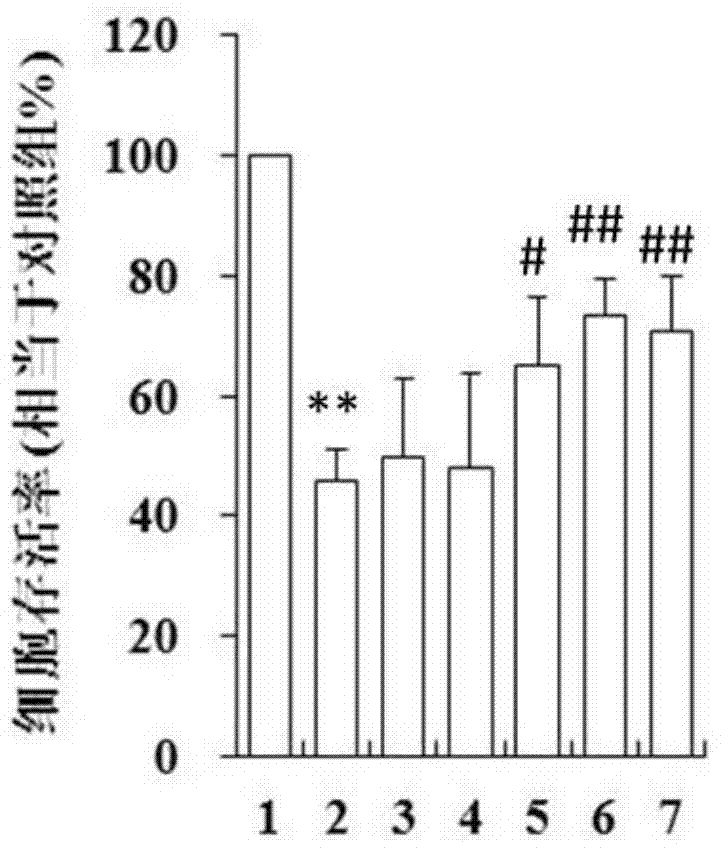
[0037] Experimental Example 1 The protective effect of exogenous NADPH on primary cultured endothelial cells HUVEC
[0038] (1) Experimental materials:
[0039] The primary cultured endothelial cells HUVEC cells used in the experiment were purchased from ATCC cell bank in the United States. Cryopreservation conditions: 2ml cryopreservation tubes, 1.6 million cells per tube, containing 70% high glucose DMEM, 20% domestic fetal bovine serum, 10% DMSO.
[0040] (2) Experimental plan:
[0041] Endothelial cell HUVEC culture: culture conditions: 37°C (5% CO2, 95% air), saturated humidity, high-sugar DMEM medium, medium containing 100U penicillin and 100U streptomycin per liter; 10% domestic fetal bovine serum; The cells were grown to about 80-90% confluence, digested with trypsin-EDTA solution and then passaged, passage density: 5×10 5 Each bottle is passaged every 2-3 days; take the logarithmic growth of HUVEC cells, add an appropriate amount of trypsin-EDTA digestion solution to...
experiment example 2
[0045] Experimental Example 2 Prophylactic administration of NADPH to reduce blood-brain barrier-related immune cell responses after brain injury
[0046] (1) Experimental materials
[0047] Clean-grade male ICR mice, weighing 23-28 g, were provided by the Experimental Animal Center of Soochow University, experimental animal production license number: XCYK (Su) 2002-2008, experimental animal use license number: SYXK (Su) 2002-0037. C57BL6 and Tg(Itgax-Venus)1Mnz mice, weighing 22-27g, are male. Tg(Itgax-Venus)1Mnz mice are products of MGI Company. The mice are positive for CD11c-eYFP and are used to study the involvement of dendritic cells in the immune response after brain injury. The room temperature is 22°C, the humidity is 50-60%, the ventilation is good, artificial day and night (12h / 12h), free to eat and drink. Before the experiment, the male mice were acclimatized in the breeding environment for 2 days. Dextran-Texas (Red): product of Invitrogen Company, NADPH reagen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com