Solid-phase synthesis of goserelin
A technology of solid-phase synthesis and lysing solution, applied in the field of solid-phase synthesis of goserelin, can solve the problems of difficulty in scale-up production, side reactions, inability to determine the degree of coupling reaction and coupling effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
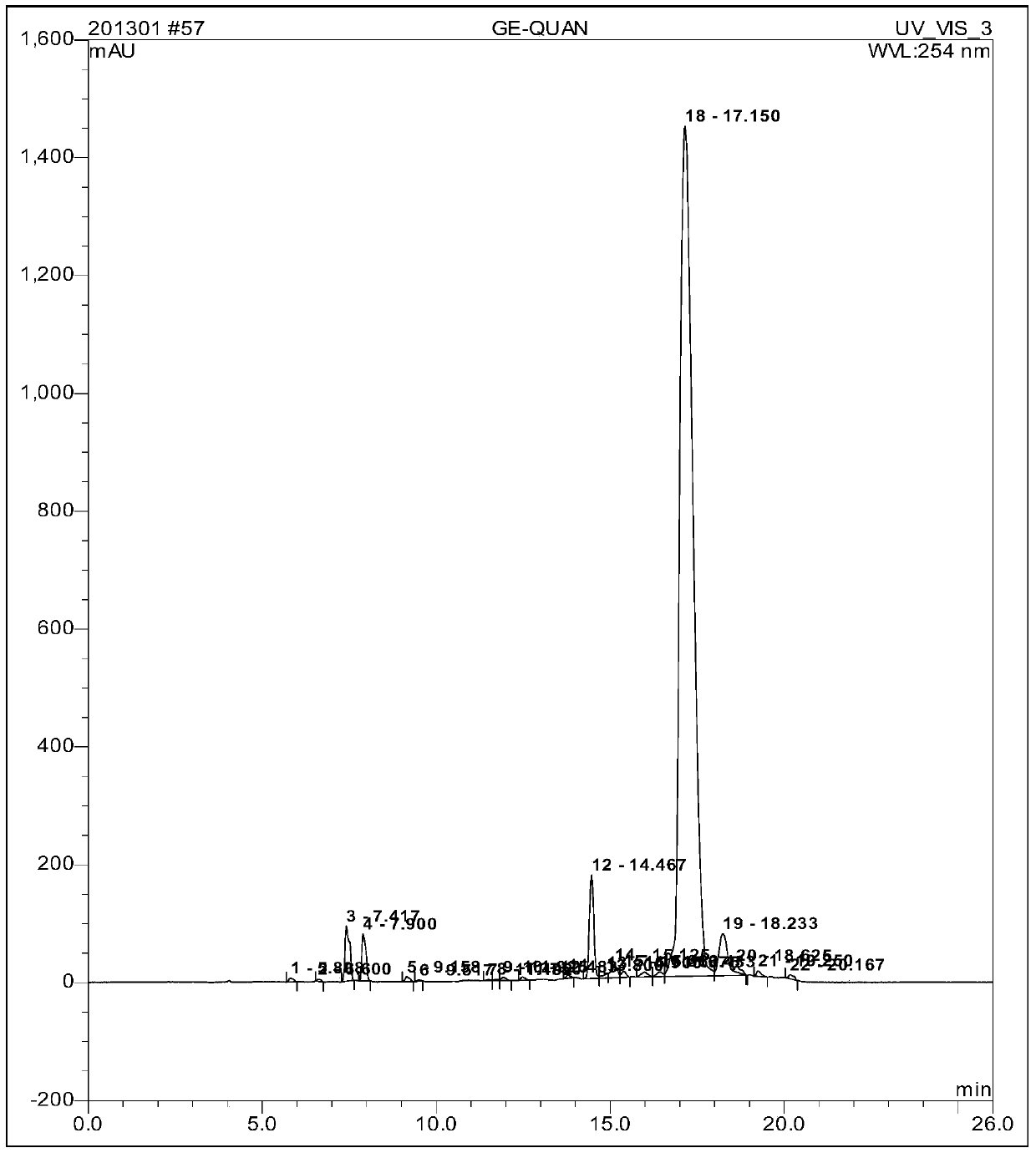
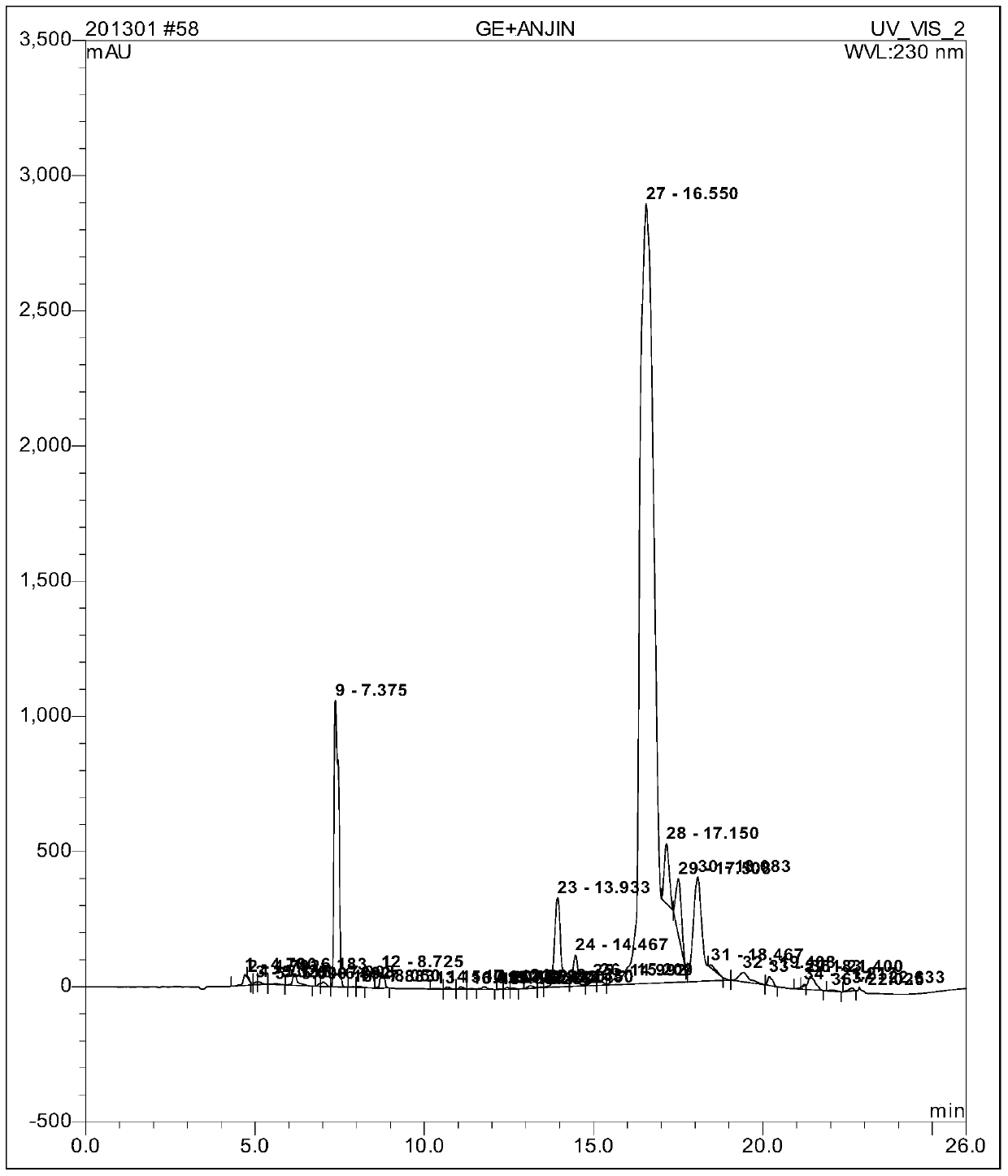
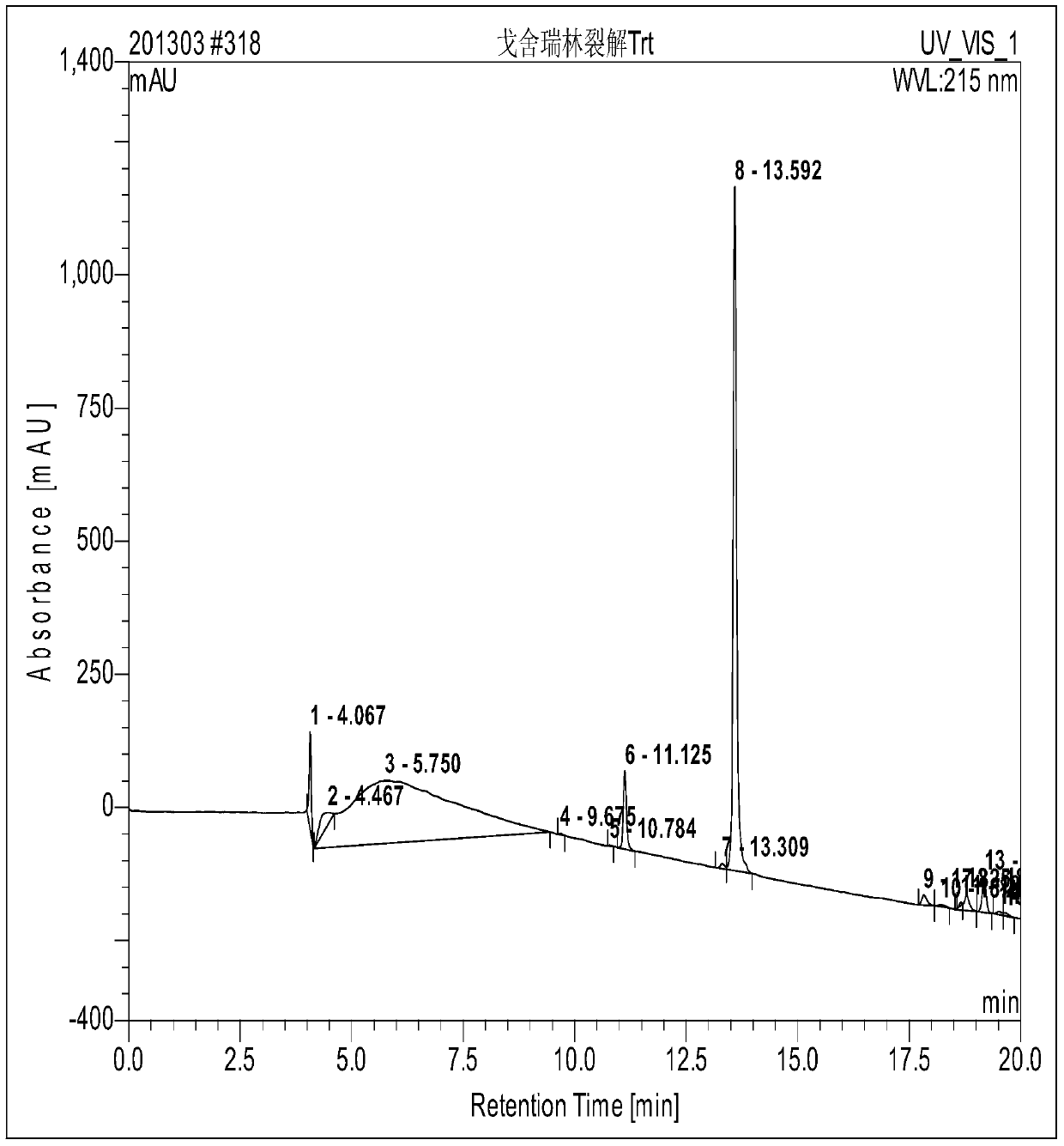
[0048] Embodiment: a kind of solid-phase synthesis method of goserelin comprises the following steps:
[0049] Step 1. According to the preset substitution degree sub (0.6mmol / g) = n (synthesis scale) / (X (裸2-CTC Resin+n* M) Molecular weight of amino acid here refers to the molecular weight of Fmoc-Pro-OH) can be calculated X (mass of naked 2-CTC Resinz) = 266g Fmoc-Pro-OH is calculated as 1.3 times the molar amount in the step of synthesizing the resin It is obtained that Fmoc-Pro-OH is 87g. Weigh 266g of 2-CTC Resin (synthesis scale: 200mmol) with a substitution degree of 1.1~1.3mmol / g and add it to the reaction column while adding 3000ml DMF to swell for 20min. Wash twice, 1min each time. Add the weighed Fmoc-Pro-OH87g to the mixed solution of DMF / DCM under the condition of ice bath, add twice the molar amount of DIPEA (N, N-diisopropylethylamine) Under the action, it reacts at room temperature, and the reaction time is determined by the expected degree of substitution. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com