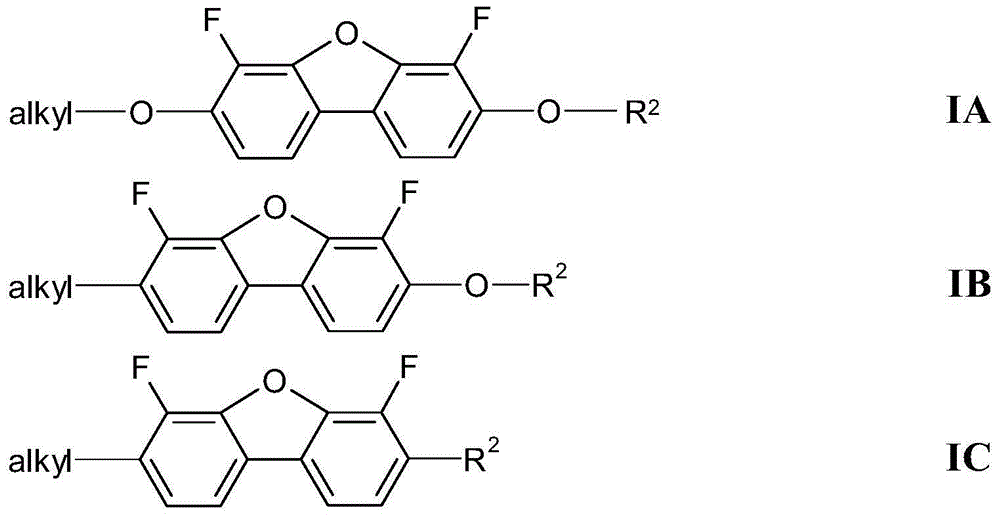
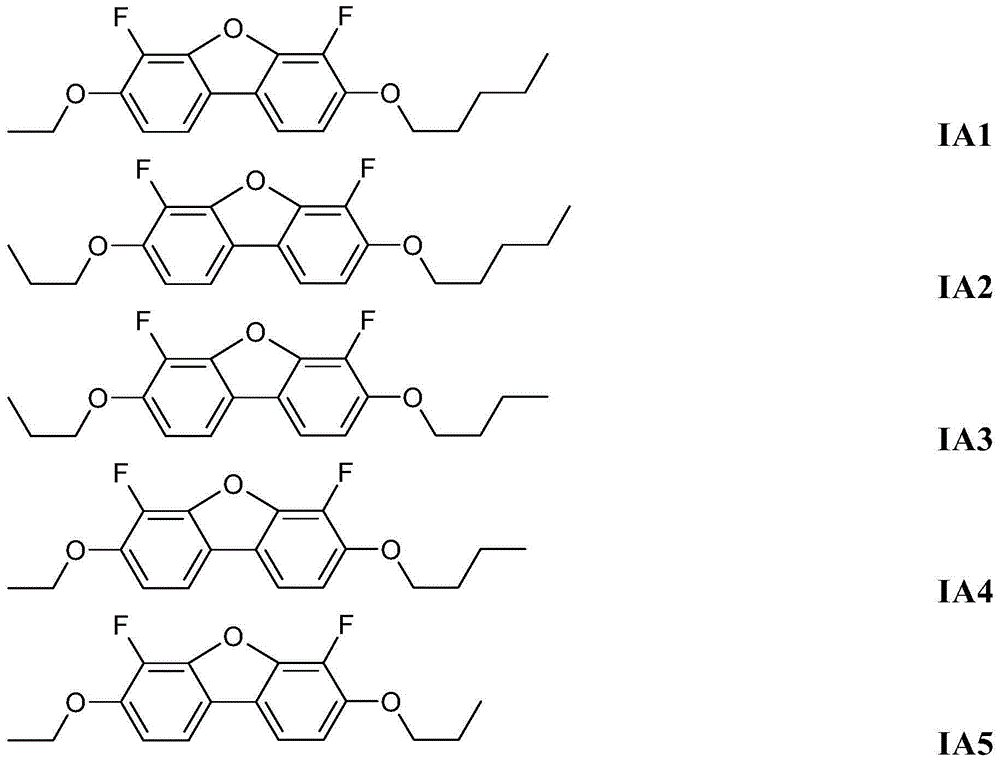
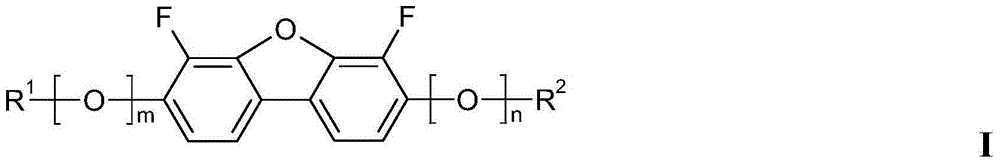4,6-difluorodibenzofuran derivatives
A compound and atomic technology, applied in the field of 4,6-difluorodibenzofuran derivatives, can solve problems such as unfinished research and development in the field of liquid crystal materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Example 1: 3-butoxy-4,6-difluoro-7-propoxydibenzofuran
[0117]
[0118] step 1
[0119]
[0120] Initially 50 g of dibenzofuran was introduced into 1500 ml of THF, and 152 g of 15% BuLi in hexane were added dropwise at -60 to -75°C. The mixture was warmed to room temperature and stirred for an additional 3 hours. The mixture was subsequently recooled to -75°C and a solution of 112.5 g of N-fluorobenzenesulfonimide in 1000 ml of THF was added at -75 to -60°C. After an additional 30 minutes at -70°C, the reaction mixture was allowed to warm to ambient temperature, and the batch was hydrolyzed with water and subjected to extractive workup.
[0121] The crude product (red-brown oil) was purified by chromatography (eluent: n-heptane).
[0122] white crystals.
[0123] step 2
[0124]
[0125] Initially 40 g of 4-fluorodibenzofuran was introduced into 450 ml of THF, and 96 g of 15% BuLi in hexane were added dropwise at -60 to -75°C. The mixture was stirred ...
Embodiment 1 and approach 2
[0166] The following compounds were prepared analogously to Example 1 and Scheme 2:
[0167]
[0168] Unless otherwise specified, R 1 / 2 The groups are linear, ie unbranched. In Table 2 the substance data are given.
[0169] Table 2
[0170]
[0171]
[0172]
[0173] *) trans isomer
Embodiment 1 and approach 3
[0174] The following compounds were prepared analogously to Example 1 and Scheme 3:
[0175]
[0176] Unless otherwise specified, R 1 / 2 The groups are linear, ie unbranched. In Table 3 the substance data are given.
[0177] table 3
[0178]
[0179]
[0180] *) trans isomer
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com