Lung cancer and breast cancer treatment compound preparation containing active ingredients of traditional Chinese medicine and preparation method of lung cancer and breast cancer compound preparation
A technology of active ingredients and compound preparations, applied in the field of compound preparations of active ingredients of traditional Chinese medicine for the treatment of lung cancer and breast cancer and its preparation, can solve the problems of large toxic and side effects, unsatisfactory curative effect, and low curative effect of cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
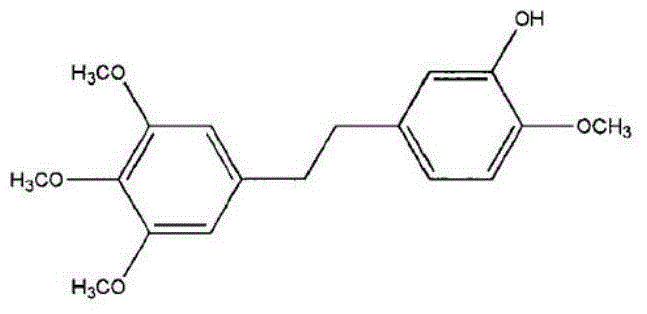
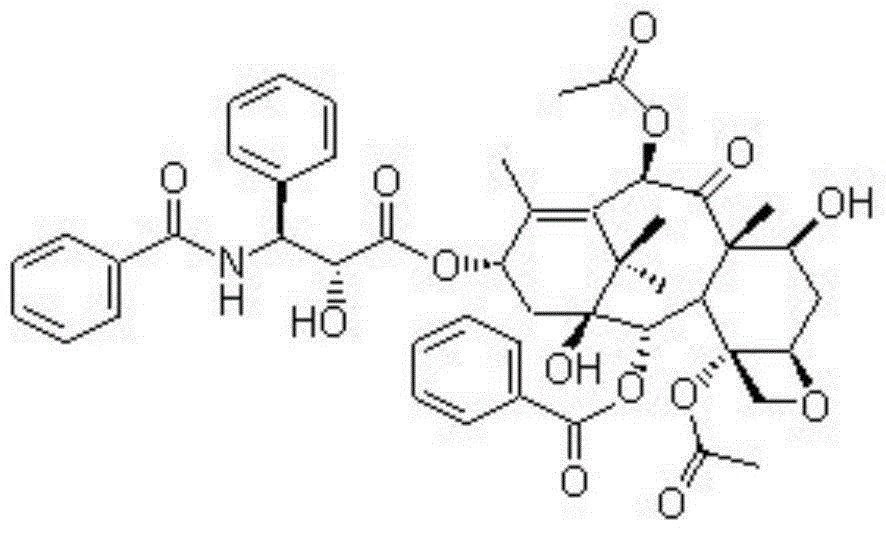
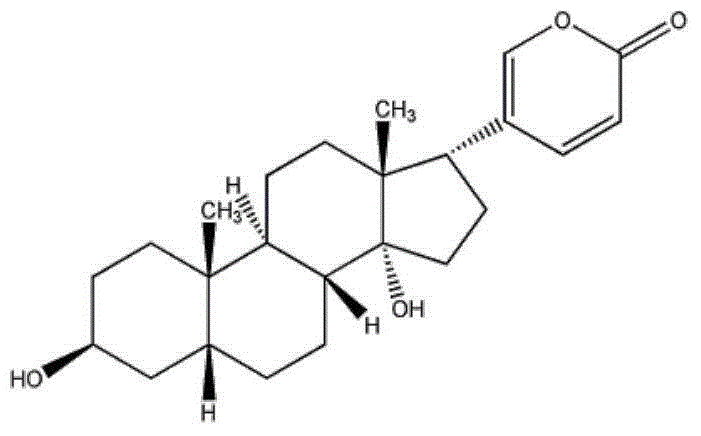
[0027] Embodiment 1: A kind of active ingredient compound preparation of Chinese medicine for the treatment of lung cancer and breast cancer, consisting of 1 part (10 mg) of erianin, 1 part (10 mg) of taxol, 1 part (10 mg) of bufoling (10 mg) and brucein D1 Part (10mg) is made, wherein the preparation method of liposome is as follows:
[0028] (1) The preparation materials and proportions of the active ingredient compound liposome of traditional Chinese medicine are as follows: the mass ratio of lecithin to mPEG2000-DSPE (amphipathic polyethylene glycol 2000-distearoylphosphatidylethanolamine) is 10:1, and The mass ratio of cholesterol is 10:1, and the mass ratio of the active ingredient compound drug of traditional Chinese medicine is 30:1.
[0029] (2) The preparation method of the compound liposome active ingredient of traditional Chinese medicine is as follows: prepare by ethanol injection method, accurately measure 90mL of PBS buffer solution with pH 7.4 in an eggplant-sh...
Embodiment 2
[0033] Embodiment 2: a kind of Chinese medicine active ingredient compound preparation for the treatment of lung cancer and breast cancer, by Erianin 2 parts (20mg), taxol 2 parts (20mg), bufalin 2 parts (20mg) and brucein D2 Part (20mg) is made, wherein the preparation method of liposome is as follows:
[0034] (1) The preparation materials and proportions of the active ingredient compound liposome of traditional Chinese medicine are as follows: the mass ratio of lecithin to mPEG2000-DSPE (amphipathic polyethylene glycol 2000-distearoylphosphatidylethanolamine) is 10:1, and The mass ratio of cholesterol is 10:1, and the mass ratio of the active ingredient compound drug of traditional Chinese medicine is 30:1.
[0035] (2) The preparation method of the compound liposome active ingredient of traditional Chinese medicine is as follows: prepare by ethanol injection method, accurately measure 180mL of PBS buffer solution with pH 7.4 in an eggplant-shaped bottle, preheat in a const...
Embodiment 3
[0039] Embodiment 3: a kind of Chinese medicine active ingredient compound preparation for the treatment of lung cancer and breast cancer, by Erianin 3 parts (30mg), taxol 3 parts (30mg), bufoling 3 parts (30mg) and brucein D3 Part (30mg) is made, wherein the preparation method of liposome is as follows:
[0040] (1) The preparation materials and proportions of the active ingredient compound liposome of traditional Chinese medicine are as follows: the mass ratio of lecithin to mPEG2000-DSPE (amphipathic polyethylene glycol 2000-distearoylphosphatidylethanolamine) is 10: 1.2, and The mass ratio of cholesterol is 10:1.2, and the mass ratio of the active ingredient compound drug of traditional Chinese medicine is 30:0.8.
[0041] (2) The preparation method of the compound liposome active ingredient of traditional Chinese medicine is as follows: prepare by ethanol injection method, accurately measure 270mL of PBS buffer solution with pH 7.4 in an eggplant-shaped bottle, preheat in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com