Difluprednate emulsion composition containing antimicrobial metal
The technology of an emulsion composition and difluprednate, which is applied in the directions of drug combination, emulsion delivery, medical preparations containing active ingredients, etc., can solve the problem of high risk of contamination of the drug solution by opening the cap.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
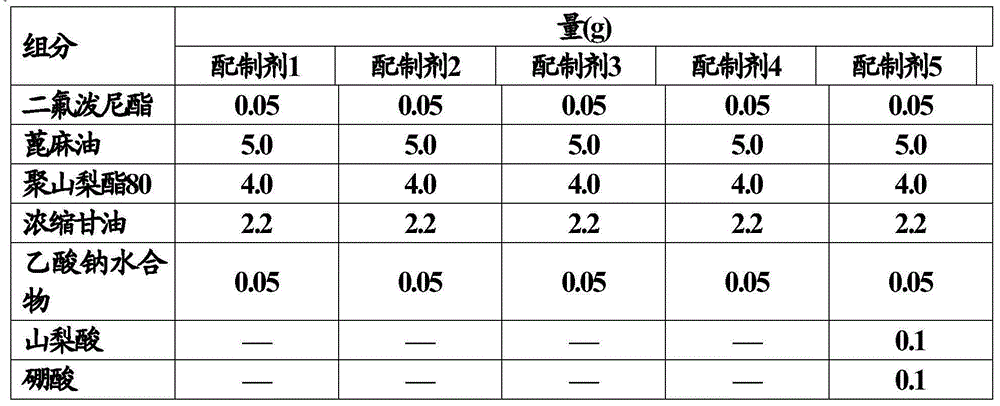
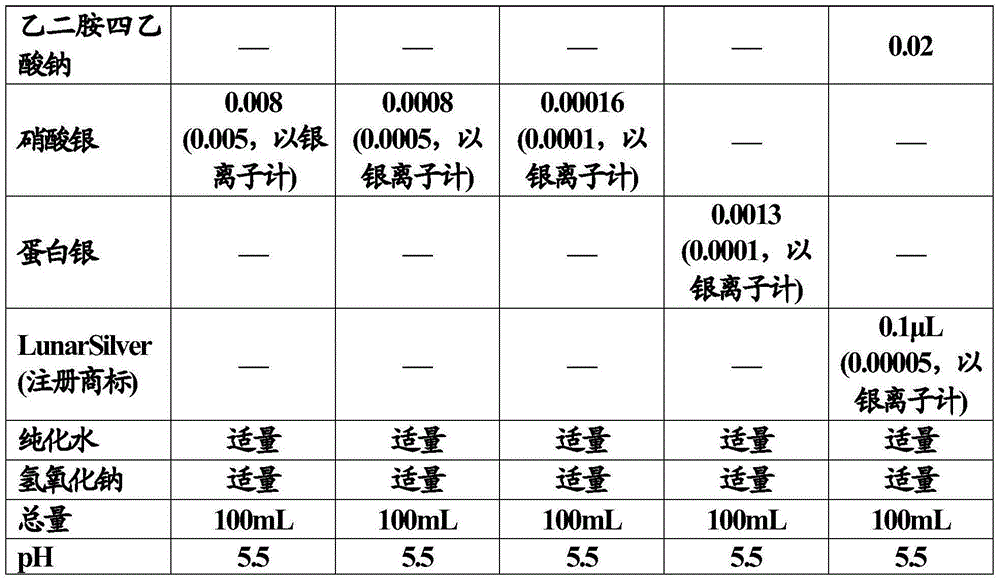
[0080] In the preparation step of the composition of the present invention, antimicrobial metals (except zinc) and additives such as tonicity agents, buffers, etc. can be dissolved in the water phase, or added to the emulsion after emulsification.
[0081] The composition of the present invention is preferably used as a topical preparation for the eyes, nose, ear or skin, and further used as an ophthalmic composition such as eye drops, nasal drops, ear drops or lotions.
[0082] The composition of the present invention has excellent anti-inflammatory effect, excellent anti-allergic effect and excellent antimicrobial effect. Therefore, the composition can be used to prevent or treat various inflammatory diseases or allergic diseases, such as allergic conjunctivitis, vernal conjunctivitis, blepharitis, catarrhal conjunctivitis, uveitis, and those caused by eye surgery Inflammation or pain, macular edema, etc. In addition, the composition can also be advantageously used for topical a...
Embodiment
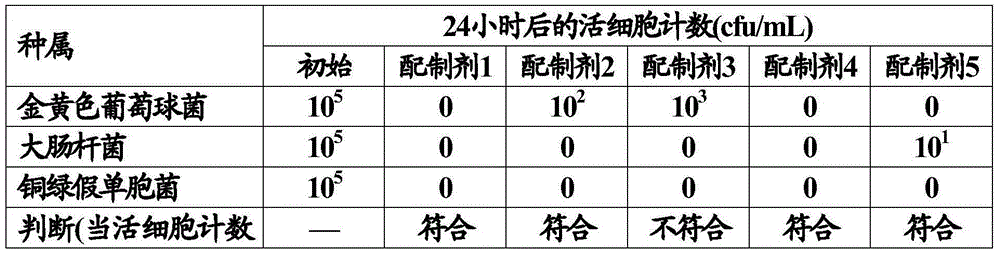
[0103] The antiseptic effect of the difluprednisolone emulsion composition was studied.
[0104] 1.1 Test method
[0105] As the test microorganisms, Staphylococcus aureus (ATCC6538), Escherichia coli (ATCC8739), and Pseudomonas aeruginosa (ATCC9027) were used. Each test microorganism was inoculated on the surface of the inclined agar medium and pre-cultured. The pre-cultivation is carried out at 30-35°C for 18-24 hours using soybean casein digested agar medium.
[0106] Using the emulsion composition to be tested as a sample, the sample was dispensed into 5 sterile capped test tubes, each of 10 mL. Add pre-cultured test microorganisms to the sample so that the cell count is 10 5 -10 6 Cells / mL to obtain a mixed sample. The test microorganisms are added separately to the sample. Store the mixed sample under light protection at 20-25°C.
[0107] At 6 hours and 24 hours from the start of storage, 1 mL of each mixed sample was measured, and the solution was diluted with saline (9 mL)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| osmolarity | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com