Substituted (e)-n'-(1-phenylethylidene) benzohydrazide analogs as histone demethylase inhibitors
A compound, C1-C6 technology, applied in the field of compounds that modulate LSD activity, compound synthesis, and inhibit LSD activity
Active Publication Date: 2016-05-04
UNIV OF UTAH RES FOUND
View PDF4 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Further, there is a scarcity of compounds that are therapeutically effective in the treatment of cancer and other diseases associated with dysfunction of LSD1 and / or LSD2
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
preparation example Construction
[0261] 2. Drug Preparation
[0262] In one aspect, the present invention relates to a method for the manufacture of a medicament for inhibiting the activity of histone demethylase in mammals, comprising combining a therapeutically effective amount of a disclosed compound or product prepared by the disclosed method and a pharmaceutically acceptable carrier or thinner.
[0263] F. Experiment
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
Substituted (E)-N-(1-phenylethylidene)benzohydrazide analogs or (3-(5-chloro-2-hydroxyphenyl)-4-METHYL-1 H-pyrazol-1-YL)(3- (morpholinosulfonyl)phenyl)methanone, derivatives thereof, and related compounds are useful as inhibitors of lysine-specific histone demethylase, including LSD1. Synthetic methods for making the compounds; pharmaceutical compositions comprising the compounds; and methods of using the compounds and compositions to treat disorders associated with dysfunction of the LSD1 (lysine-specific demethylase). A pharmaceutically acceptable salt, hydrate, solvate, or polymorph thereof, and one or more of: (a) at least one agent known to increase histone demethylase activity; (b) at least one agent known to decrease histone demethylase activity; (c) at least one agent known to treat a disorder of uncontrolled cellular proliferation; (d) at least one agent known to treat a neurodegenerative disorder; (e) instructions for treating a neurodegenerative disorder; or (f) instructions for treating a disorder associated with uncontrolled cellular proliferation.
Description
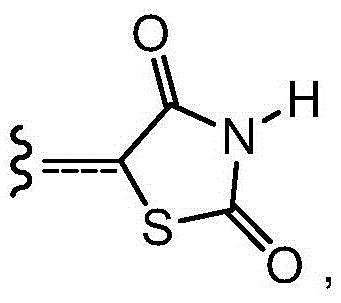
Background technique [0001] In the last decade, it has become evident that epigenetic changes, which alter the activity of genes without altering the DNA sequence, together with genetic errors, contribute to the development and progression of cancer (Tsai, H.C. and Baylin, S.B. CellRes 2011, 21(3), 502-17; and Fullgrabe, J., Kavanagh, E., and Joseph, B. Oncogene 2011). The regulation of modifications to DNA and DNA-associated proteins has become an area of intense interest, and enzymes involved in these processes have been suggested as a new class of protein targets for drug development. The major proteins associated with DNA are histones. The tails of histones host various post-translational modifications, such as phosphorylation, acetylation, methylation, and ubiquitination, and these modifications, especially acetylation and methylation of lysine residues, are important in the regulation of gene expression. plays a major role in and is often dysregulated in cancer (Full...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D409/12C07D403/12C07D403/14C07D401/14A61K31/506
CPCC07D213/87C07D231/12C07D211/96C07D295/26A61P35/00A61P43/00
Inventor 哈里拉萨德·梵卡亚拉帕蒂文卡塔斯瓦米·索尔纳史蒂文·L·瓦尔奈戴维·J·贝尔斯苏尼尔·沙玛布雷特·斯蒂芬斯
Owner UNIV OF UTAH RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com