Fluorine-substituted monocarbazole derivative, and preparation method and application thereof
A technology for monocarbazole derivatives, applied in the field of its preparation, fluorine-substituted monocarbazole derivatives, can solve the problems of low selectivity, poor activity, and affecting the overall stability of chromosomes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Synthesis and characterization of embodiment 1 compound 1
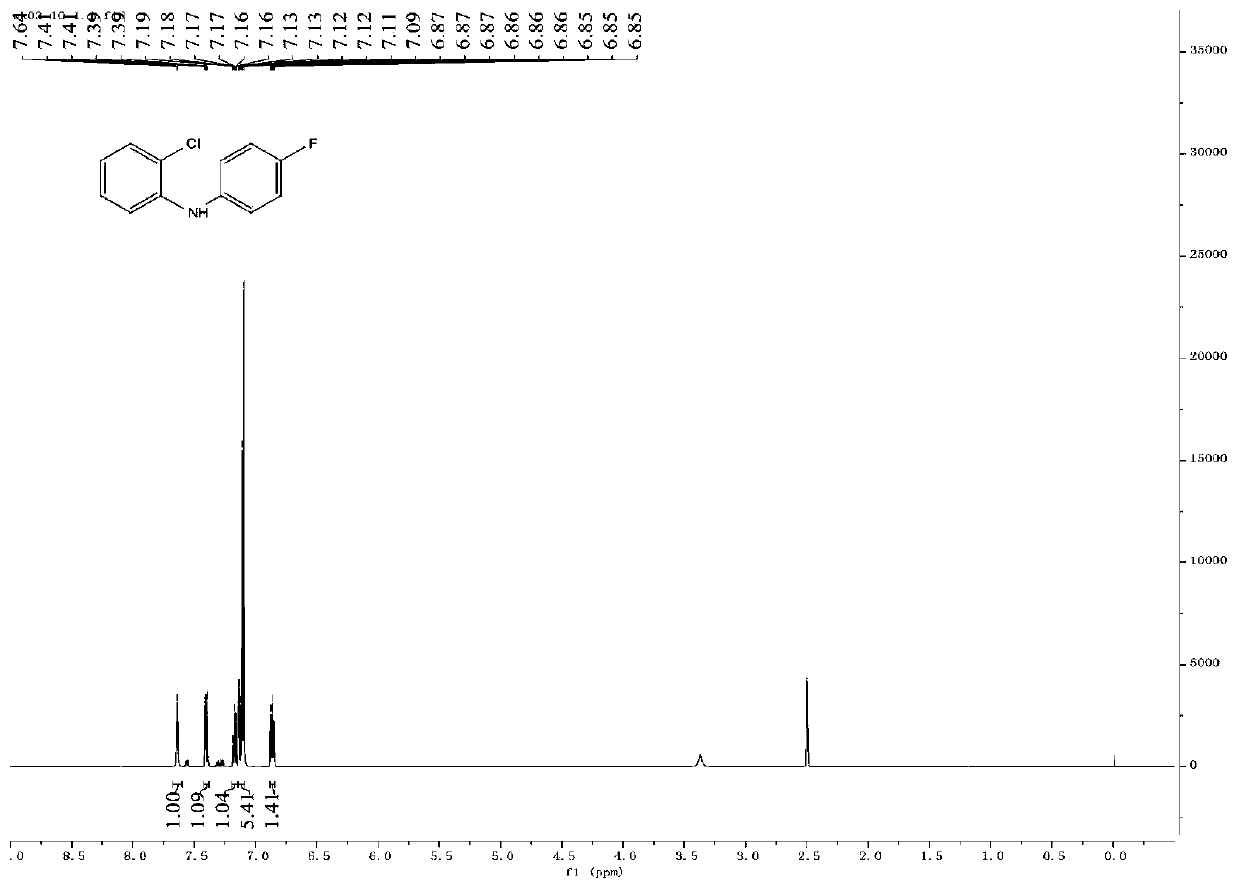
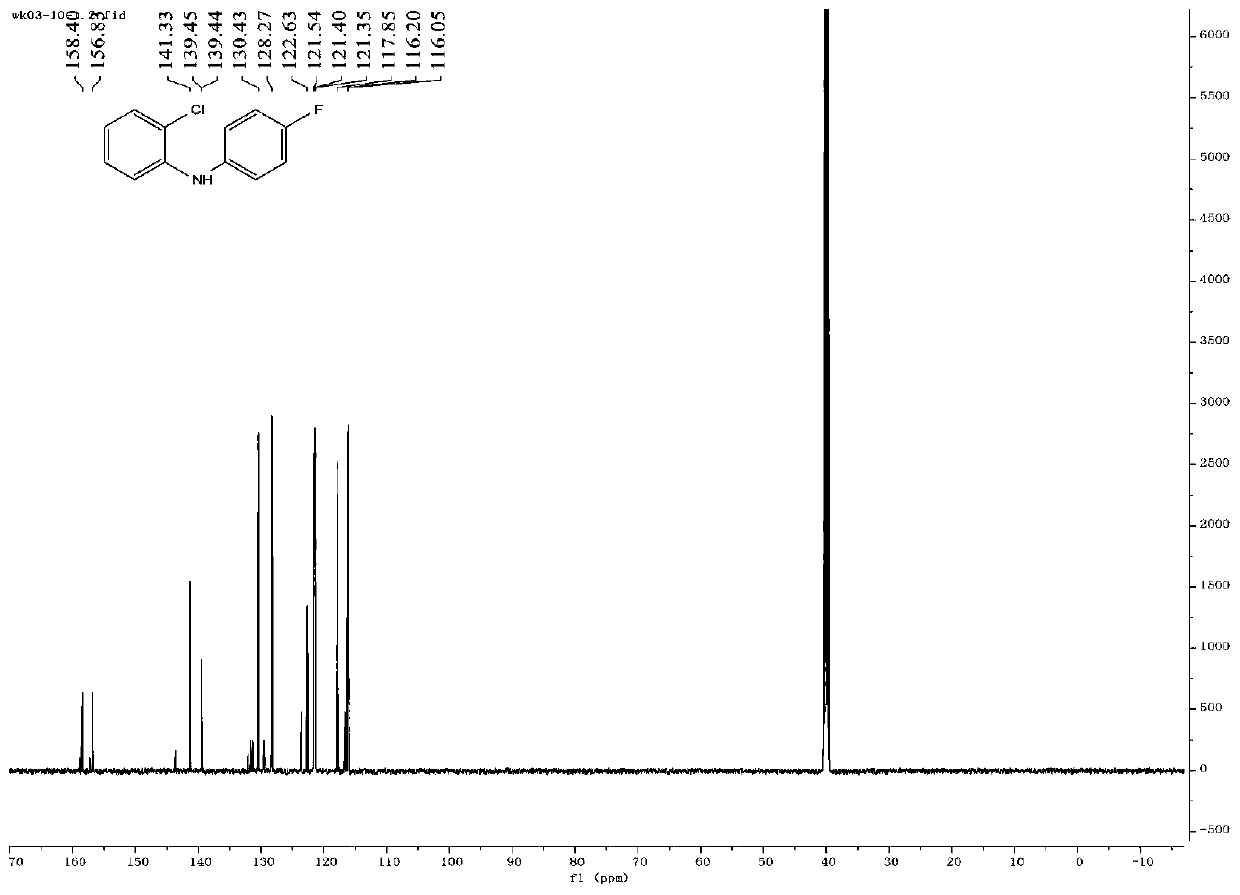
[0129] Take 120.0mg (0.5mmol) of compound a4 into a 10mL sealed tube, add 5mL of absolute ethanol, add 318.7mg (2.0mmol) of tryptamine, and heat at 60°C for reaction. TLC detection (PE:EA:MeOH=5:20:2 developed) the reaction is complete, add water, extract with ethyl acetate, the organic phase is washed with sat.NaCl, anhyd.Na 2 SO 4 After drying, the solvent was distilled off under reduced pressure to obtain 0.37 g of a crude product. Purified by column chromatography (PE:EA=1:1) to obtain 64.0 mg of white solid with a yield of 32.10%. Carry out infrared spectrum, mass spectrum, hydrogen spectrum and carbon spectrum NMR and DEPT spectrum analysis to compound 1, such as Figures 17-21 As shown, the specific data of infrared spectrum, mass spectrum, hydrogen spectrum and carbon spectrum are as follows:
[0130] IR(KBr),ν,cm -1 :3273,2930,1487,1459,1439,1351,1281,1170,889,795,727.
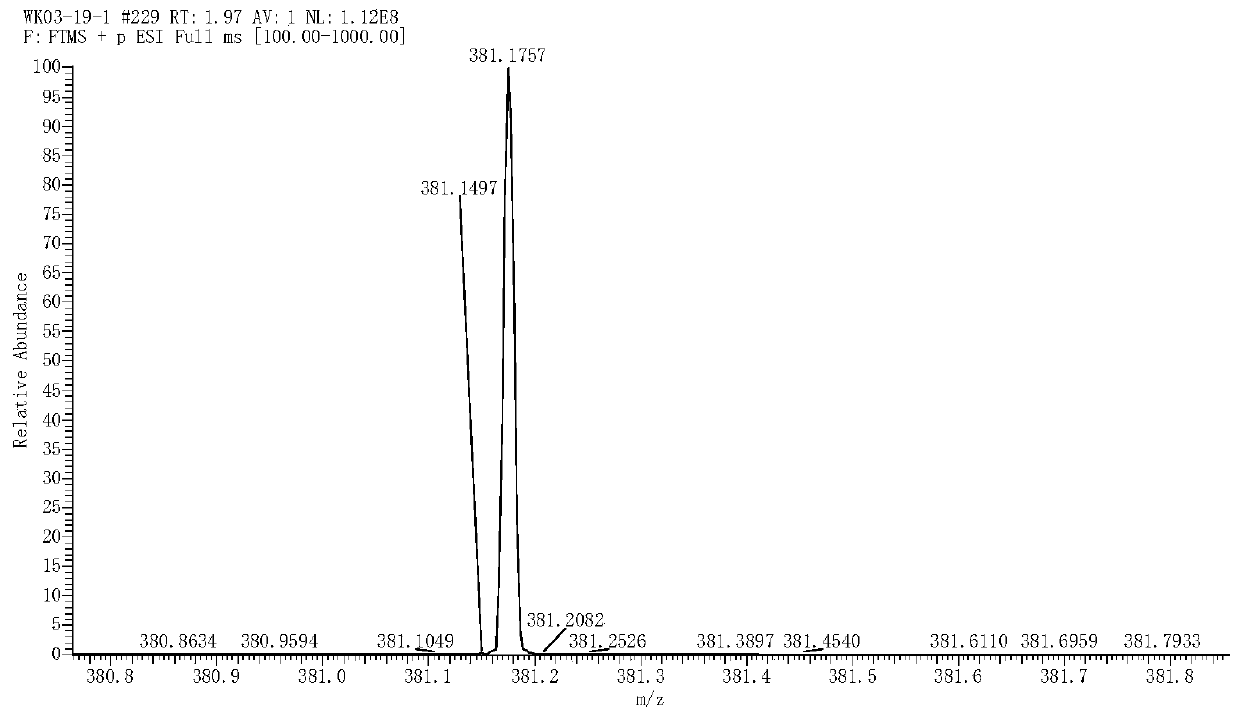
[0131] m.p.137-139℃.HRMS-...
Embodiment 2
[0135] Synthesis and characterization of embodiment 2 compound 2
[0136] Take 120.0 mg (0.5 mmol) of compound a4 into a 10 mL sealed tube, add 5 mL of absolute ethanol, add 277.0 mg (2.0 mmol) of 4-fluorophenethylamine, and heat at 60°C for reaction. TLC detection (PE:EA:MeOH=5:20:2 developed) the reaction is complete, add water, extract with ethyl acetate, the organic phase is washed with sat.NaCl, anhyd.Na 2 SO 4 After drying, the solvent was distilled off under reduced pressure to obtain 0.35 g of a crude product. Purified by column chromatography (PE:EA=1:1) to obtain 94.0 mg of white solid with a yield of 49.67%.
[0137] Carry out infrared spectrum, mass spectrum, hydrogen spectrum and carbon spectrum NMR analysis and DEPT spectrum analysis to compound 2, such as Figures 22 to 26 As shown, the specific data of infrared spectrum, mass spectrum, hydrogen spectrum and carbon spectrum NMR analysis are as follows:
[0138] IR(KBr),ν,cm -1 :3059,2822,1603,1511,1486,1465...
Embodiment 3
[0143] Synthesis and characterization of embodiment 3 compound 3
[0144] Take 158.0mg (0.7mmol) of compound a4 into a 10mL sealed tube, add 5mL of absolute ethanol, add 359.5mg (2.6mmol) of p-hydroxyphenylethylamine, and heat at 60°C for reaction. TLC detection (PE:EA:MeOH=5:20:1 development) the reaction is complete, add water, extract with ethyl acetate, the organic phase is washed with sat.NaCl, anhyd.Na 2 SO 4 After drying, the solvent was distilled off under reduced pressure to obtain 0.39 g of a crude product. Purified by column chromatography (PE:EA=1:2) to obtain 119.0 mg of white solid with a yield of 47.98%.
[0145] Carry out infrared spectrum, mass spectrum, hydrogen spectrum and carbon spectrum NMR analysis and DEPT spectrum analysis to compound 3, such as Figures 27 to 31 As shown, the specific data of infrared spectrum, mass spectrum, hydrogen spectrum and carbon spectrum are as follows:
[0146] IR(KBr),ν,cm -1 :2932,1597,1518,1488,1465,1465,1271,1245,11...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com