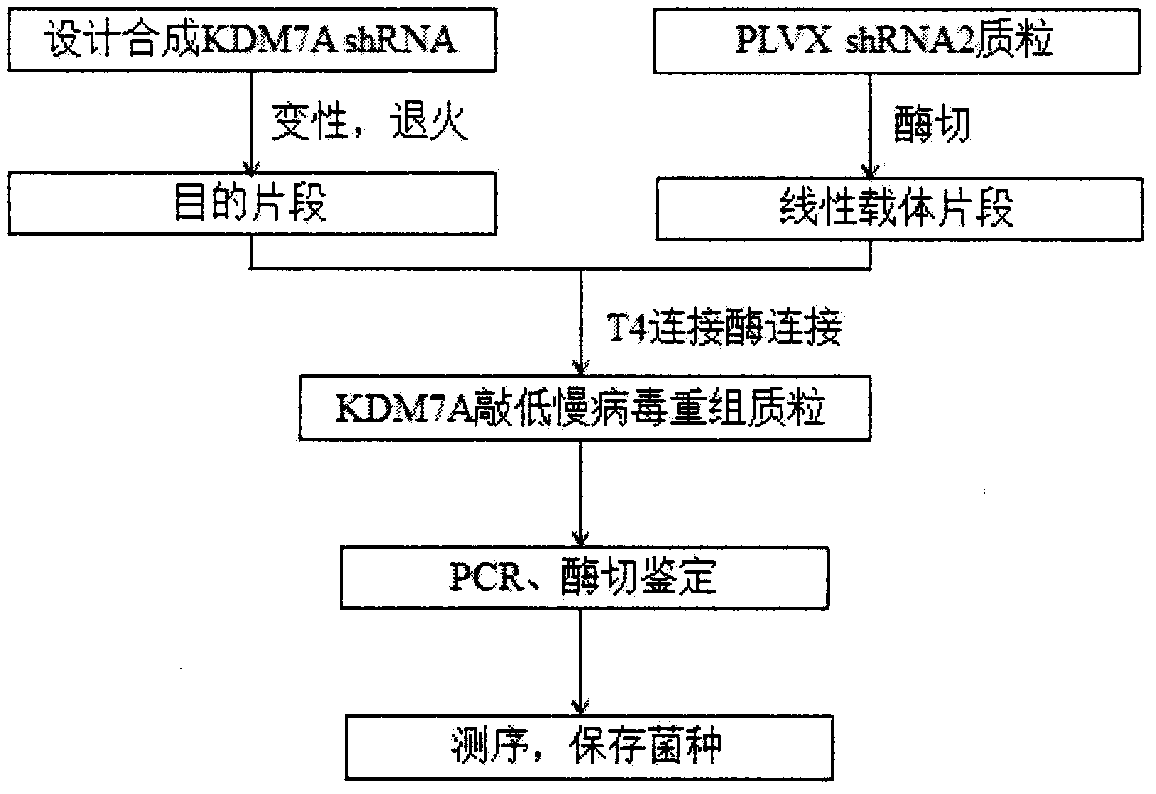
Construction of ShRNA lentiviral vector for targeted silencing of Kdm7a gene and application of shRNA lentiviral vector
A targeted silencing and lentiviral technology, applied in the fields of molecular biology and biomedicine, can solve the problems of functional research limitations, unreported and unseen research on osteogenic differentiation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Construction of shRNA lentiviral vector targeting silencing Kdm7a gene
[0029] 1. Design the shRNA sequence targeting Kdm7a silencing. The sense strand and antisense strand oligonucleotides of the Kdm7a shRNA coding sequence were synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.
[0030] 2. Synthesis of target fragments: The sense strand and antisense strand oligonucleotides were dissolved at a concentration of 50 μM, and 5 μl each was taken in a 0.2 mL EP tube for denaturation and annealing, and the reaction was carried out in a PCR instrument under the following conditions: 95°C for 1 min, 50 °C for 2 min, then cool down at room temperature.
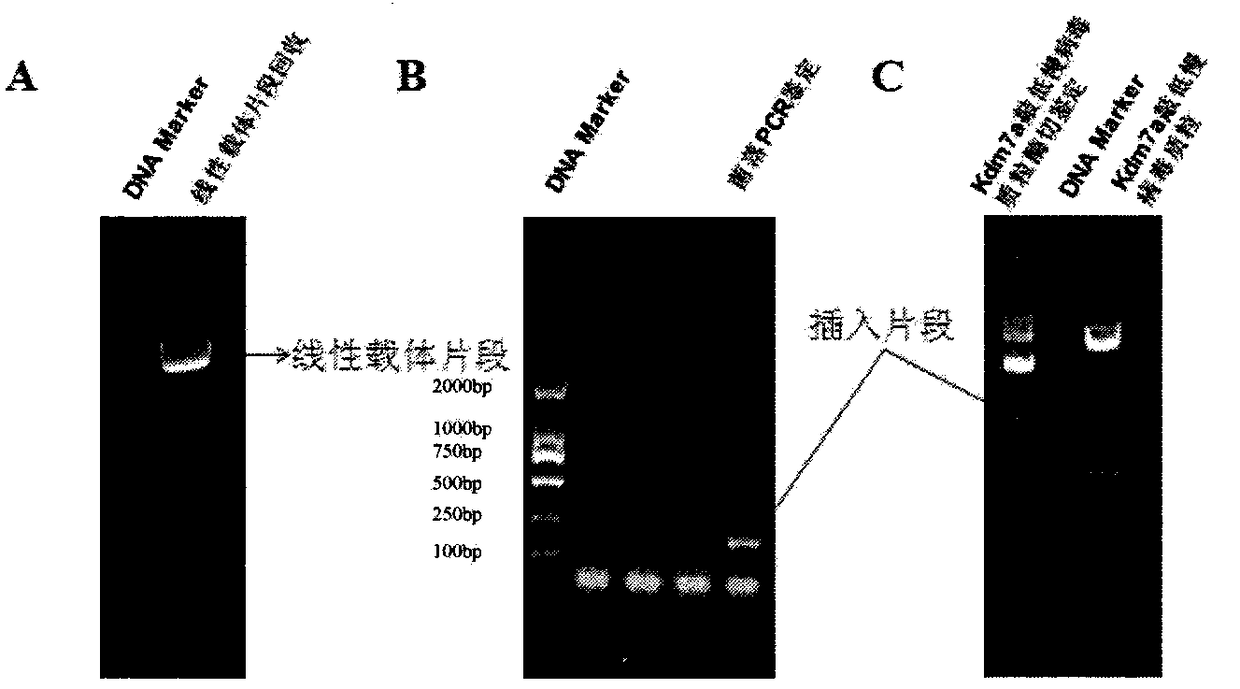
[0031] 3. Obtaining the linear vector fragment: the pLVX-shRNA2 plasmid was digested with restriction endonucleases BamHI and EcoRI, electrophoresed on 1.2% agarose gel, and the linear vector fragment was recovered.
[0032] 4. T4 ligase ligated the target fragment and the linear vector fragment, transformed...
Embodiment 2
[0041] Example 2 Packaging pLVX-Kdm7a shRNA recombinant lentivirus
[0042] 1. Recovery and passage of 293T cells: Recovery: Take 293T cells frozen in a liquid nitrogen tank, quickly place them in water at 37-38°C, and shake. After it was completely melted, centrifuge at 1000rpm for 10min at room temperature. Discard the frozen storage solution, resuspend the cells in DMEM medium containing 10% FBS and double antibodies, inoculate them into cell culture flasks, and store at 37°C, 5% CO 2 Incubate in a cell culture incubator. Every 3 days, replace the cell culture medium. Observe the state of the cells under a microscope, and when the confluence of the cells reaches 80% to 90%, they can be passaged.
[0043] 2. Cell inoculation: 24 hours before transfection, digest 293T cells with 0.25% trypsin, and inoculate into a new 10cm culture dish. When the confluence of the cells in the well plate reached 80%-90%, the empty lentiviral plasmid pLVX shRNA2 (Ctrl LV) and pLVX-Kdm7a shRNA...
Embodiment 3
[0061] Example 3 Effect of pLVX-Kdm7a shRNA recombinant lentivirus on osteogenic differentiation of mouse bone marrow mesenchymal cells
[0062] 1. Cell inoculation: 24 hours before transfection, the mouse bone marrow mesenchymal cells were digested with 0.25% trypsin, and inoculated into a 24-well plate. When the confluence of the cells in the well plate reached 30%-40%, the control lentivirus pLVX shRNA2 (Ctrl LV) and pLVX-Kdm7a shRNA recombinant lentivirus (Kdm7a-KD LV) were infected.
[0063] 2. The infection system and method are as follows:
[0064]
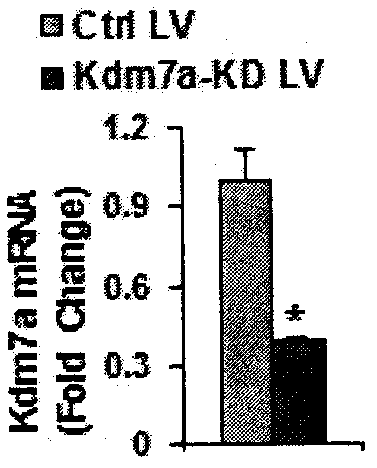
[0065] 3. Observe the state of the cells after infection, replace the medium after 24-48 hours of infection, collect the cells, extract total RNA, reverse transcribe and synthesize cDNA, and detect the expression of Kdm7a in primary mouse bone marrow mesenchymal cells after infection with lentivirus by qRT-PCR mRNA expression.
[0066] 4. Induce osteogenic differentiation and detect the mRNA expression of specific fact...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com