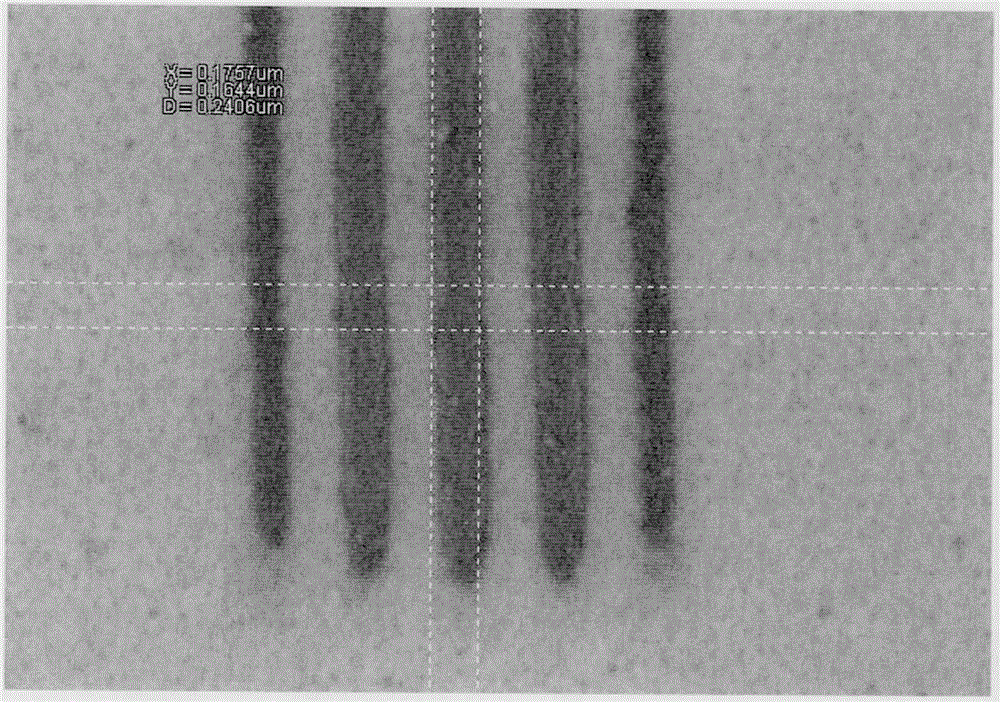
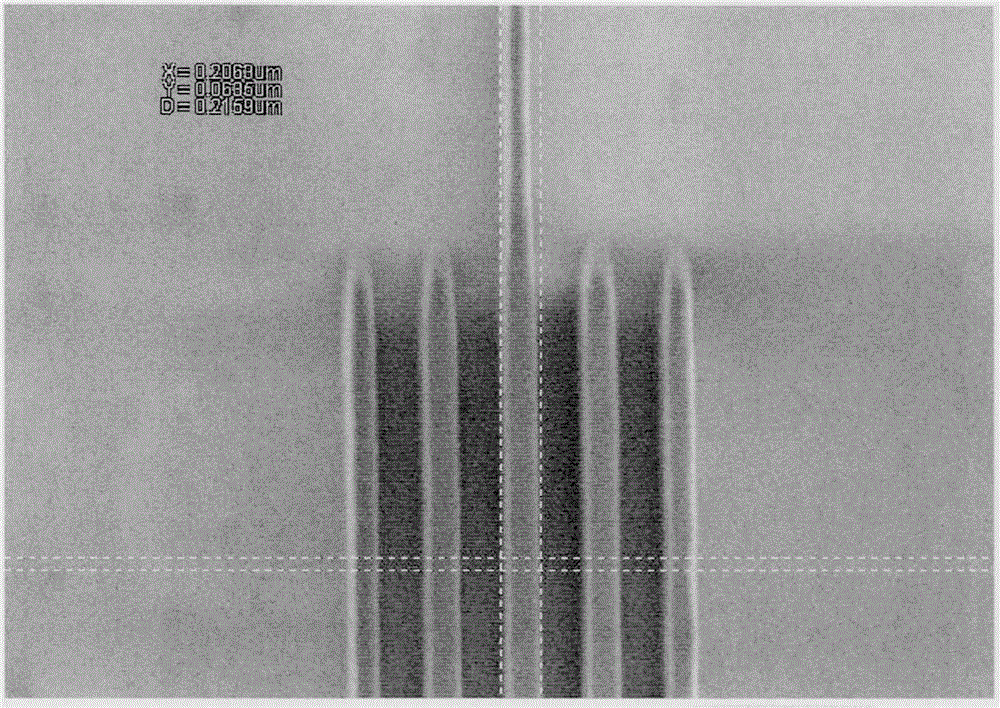
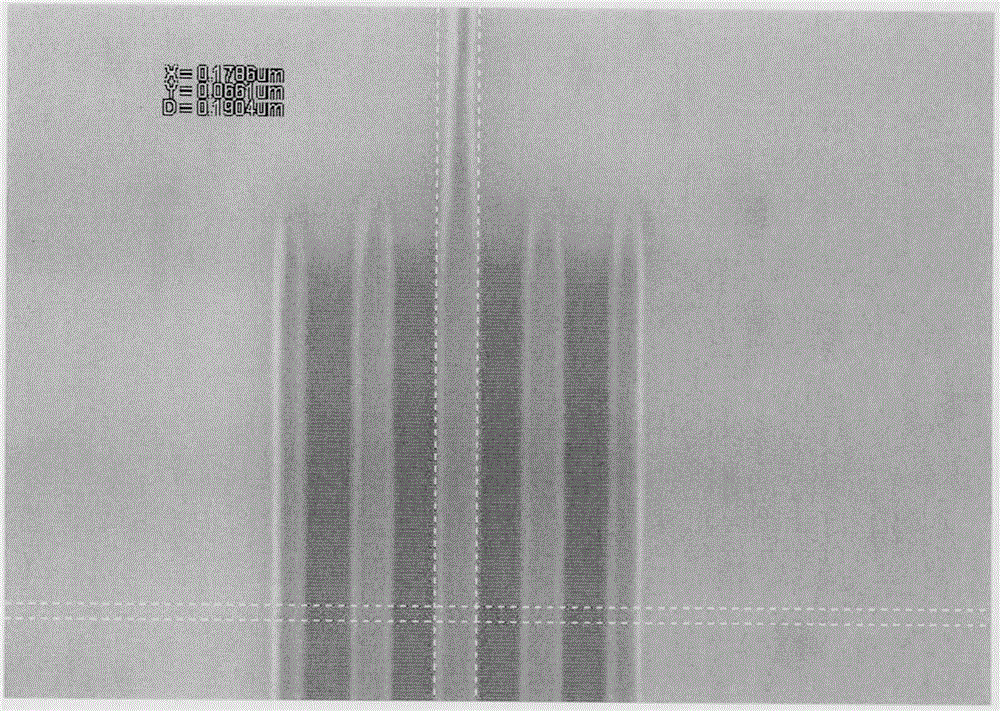
Styrene derivative-methacrylate copolymer containing photo-acid generation groups as well as preparation and application of styrene derivative-methacrylate copolymer
A technology of methacrylate and styrene derivatives, which is applied in the field of styrene derivatives-methacrylate copolymers containing photoacid generating groups, its preparation and application, and can solve the problem of increasing the roughness of line edges , Uneven distribution of acid production, lower graphics resolution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Preparation of Polymer P (SSS-HS (35% BOC)-HEMA) Containing Photoacid Generating Groups (The Ratio of Monomers is 0.6:8:1.4)
[0051] (1) Polymerization of sodium styrene sulfonate (NaSS), acetoxystyrene (AoS) and hydroxyethyl methacrylate (HEMA)
[0052] NaSS (2.47g, 0.012mol), AoS (25.9g, 0.16mol) and HEMA (3.65g, 0.028mol) were added to a 250mL four-neck flask equipped with a spherical condenser, a thermometer and a magnetic stirrer, and 100mL of solvent was added DMSO. Feed nitrogen into the system as a reaction protection gas, and heat the oil bath to react, the reaction temperature is controlled at 75-85 ° C, and the molecular weight regulator n-dodecanethiol (0.32 g, 1wt%) and initiator AIBN (1.64g, 5% mol), react 7-8h, stop heating, and the temperature of the reaction is down to room temperature. The infrared spectrum of the polymerization product P (NaSS-AoS-HEMA) is shown in the appendix figure 1 .
[0053] (2) Hydrolysis reaction
[0054] Add 11...
Embodiment 2
[0063] Example 2 Preparation of Polymer P(PSS-HS(35%BOC)-tBMA) Containing Photoacid Generating Groups (The Ratio of Monomers is 0.9:8:1.1)
[0064] (1) Polymerization of sodium styrene sulfonate (NaSS), acetoxystyrene (AoS) and tert-butyl methacrylate (tBMA)
[0065] NaSS (3.71g, 0.018moD, AoS (25.9g, 0.16mol) and tBMA (3.12g, 0.022mol) were added to a 250mL four-neck flask equipped with a spherical condenser, a thermometer and a magnetic stirrer, and 100mL of solvent DMSO was added .Introduce nitrogen into the system as the reaction protection gas, and heat the oil bath to react, the reaction temperature is controlled at 75-85°C, and the molecular weight regulator n-dodecanethiol (0.32g , 1wt%) and initiator AIBN (1.64g, 5% mol), react 7-8h, stop heating, wait for the reaction temperature to drop to room temperature. The infrared spectrum of polymerization product P (NaSS-AoS-tBMA) sees attached image 3 .
[0066] (2) Hydrolysis reaction
[0067] Add 11.6g of concentrated...
Embodiment 3
[0079] Example 3 Preparation of Polymer P(SSS-HS(35%BOC)-DCPMA) Containing Photoacid Generating Groups (The Ratio of Monomers is 1.2:8:0.8)
[0080] (1) Reaction of sodium styrene sulfonate (NaSS), acetoxystyrene (AoS) and dicyclopentyl methacrylate (DCPMA)
[0081] NaSS (4.94g, 0.024mol), AoS (25.9g, 0.16mol) and DCPMA (3.88g, 0.016mol) were added to a 250mL four-neck flask equipped with a spherical condenser, a thermometer and a magnetic stirrer, and 100mL of solvent was added DMSO. Feed nitrogen into the system as a reaction protection gas, and heat the oil bath to react, the reaction temperature is controlled at 75-85 ° C, and the molecular weight regulator n-dodecanethiol (0.32 g, 1wt%) and initiator AIBN (1.64g, 5%mol), react for 7-8h, stop heating, and wait for the reaction temperature to drop to room temperature. The infrared spectrum of the polymerization product P (NaSS-AoS-DCPMA) is shown in the appendix Figure 5 .
[0082] (2) Hydrolysis reaction
[0083] Add 1...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap