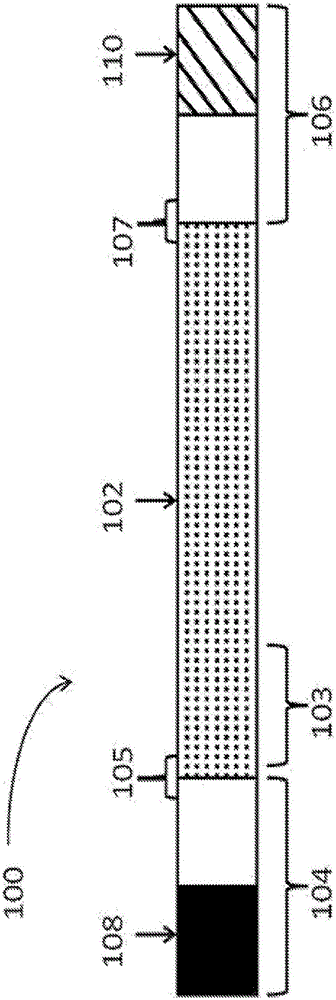
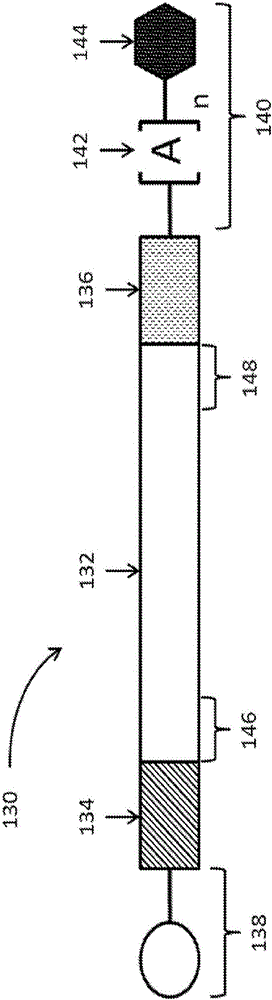
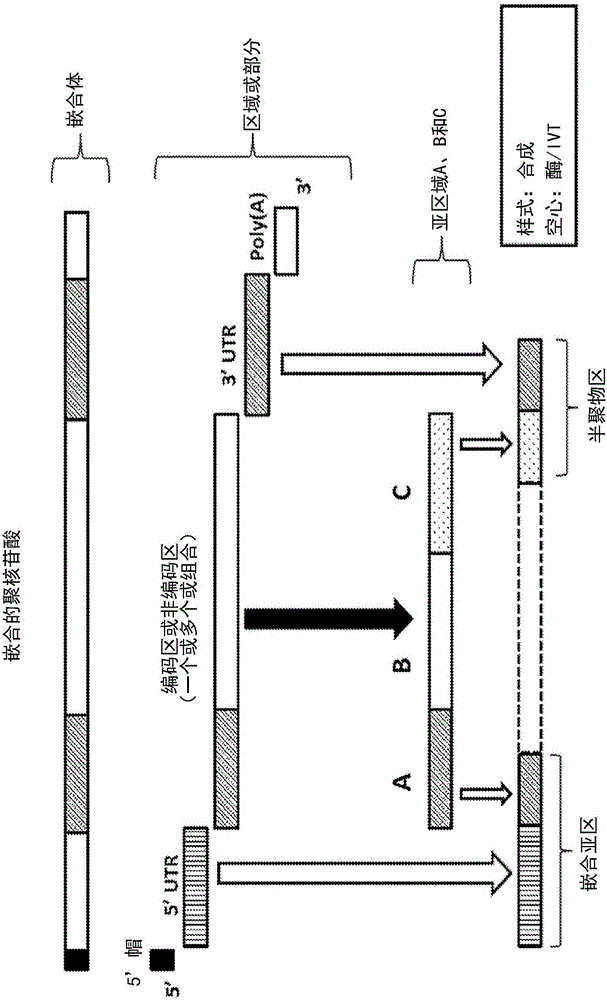
Polynucleotides encoding low density lipoprotein receptor
A low-density lipoprotein and polynucleotide technology, applied in the field of polynucleotides encoding low-density lipoprotein receptors, can solve problems such as adverse events of heart disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0491] Formulations of the pharmaceutical compositions described herein may be prepared by any method known or hereafter developed in the art of pharmacology. In general, such preparation methods comprise the steps of combining the active ingredient with excipients and / or one or more other auxiliary ingredients, and subsequently, if necessary and / or required, dividing, shaping and / or packaging the product into the desired single-dose or multiple-dose units.
[0492] The relative amounts of the active ingredients, pharmaceutically acceptable excipients and / or any additional ingredients in the pharmaceutical compositions of the present invention will vary depending on the identity, size and / or condition of the subject to be treated, and further depending on the composition to be administered path changes. By way of example, the composition may comprise between 0.1% and 100%, such as between 0.5% and 50%, between 1-30%, between 5-80%, at least 80% (w / w) active ingredient .
[...
Embodiment 1
[1205] Example 1. Production of polynucleotides
[1206] Manufacture of polynucleotides and / or portions or regions thereof in accordance with the present invention may be made using U.S. 61 / 800,049, filed March 15, 2013, entitled "Manufacturing Methods for Producing RNA Transcripts" (Attorney Docket No. M500 ), the contents of which are incorporated herein by reference in their entirety.
[1207] Purification methods may include USSN 61 / 799,872, filed March 15, 2013, entitled "Methods for Removing DNA Fragments in mRNA Production" (Attorney Docket No. M501); Those taught in USSN 61 / 794,842 (Attorney Docket No. M502) for "Purification of Ribonucleic Acids," the contents of each of which are hereby incorporated by reference in their entirety.
[1208] Methods of detection and characterization of polynucleotides may be performed as taught in U.S. 61 / 798,945 (Attorney Docket No. M505), entitled "Characterizing mRNA Molecules," filed March 15, 2013, the contents of which are inc...
Embodiment 2
[1210] Example 2. Chimeric polynucleotide synthesis: the triphosphate pathway
[1211] introduction
[1212] According to the present invention, two regions or portions of a chimeric polynucleotide can be joined or linked using triphosphate chemistry.
[1213] According to this method, a first region or portion of 100 nucleotides or less is chemically synthesized, a 5' monophosphate and a terminal 3' desOH or blocked OH. If the region is longer than 80 nucleotides, it can be synthesized as two strands for ligation.
[1214] If the first region or part is synthesized as a region or part modified in a non-positional manner using in vitro transcription (IVT), then a 5' monophosphate followed by capping of the 3' end can be followed.
[1215] The monophosphate protecting group may be selected from any of those known in the art.
[1216] The second region or portion of the chimeric polynucleotide can be synthesized using chemical synthesis or IVT methods. IVT methods can in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com