A kind of preparation method of ezetimibe and intermediate thereof
A technology of ezetimibe and an intermediate, which is applied in the field of drug synthesis, can solve the problems of complicated production process, increase production cost, increase the difficulty of operation, etc., and achieve the effects of high economic value, improved stability and reduced cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
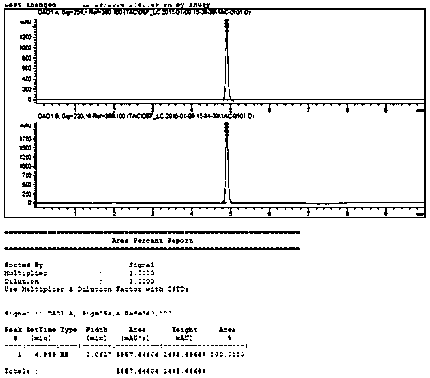
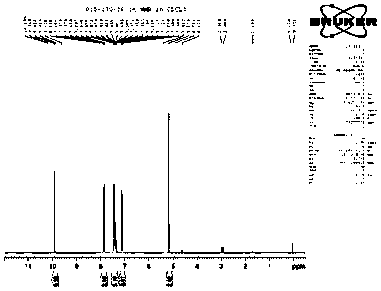
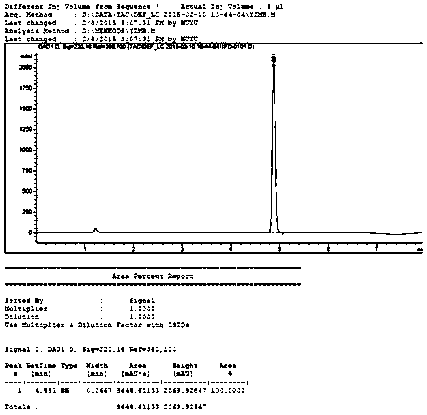
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1 4-benzyloxybenzaldehyde
[0039]
[0040] 5L four-necked flask, then stirred, added p-hydroxybenzaldehyde (100g, 1eq), benzyl chloride (124.4g, 1.2eq), potassium carbonate (135.81g, 1.2eq), N,N-dimethylformamide ( 1000ml, 10V), stirred overnight at room temperature, and monitored the end of the reaction. Filtrate, concentrate, add 1000ml of ethyl acetate and 400ml of water, extract and separate, filter and concentrate to dryness to obtain a white solid with a yield of about 97% and a purity of >99%.
Embodiment 2
[0041] The preparation of embodiment 2 compound SM2
[0042]
[0043] Take a 2L three-necked flask, stir it mechanically, add raw materials (173.7g, 1eq), p-fluoroaniline (100g, 1.1eq), isopropanol (1730ml, 10V), stir and heat to 40°C, a large amount of light yellow crystalline solids are gradually precipitated , Stir at 40°C for 2h, and monitor the end of the reaction. Stop heating and naturally stir to cool down to room temperature, filter with suction, rinse the filter cake with cold isopropanol, and dry to obtain light yellow crystal SM2 with a mass of 238 g, a yield of 95%, and a purity of 99%.
Embodiment 3
[0044] Preparation of compound (trimethylsilyl protecting group and benzyl protecting group) shown in embodiment 3 formula (III)
[0045]
[0046] 2L three-necked flask, mechanically stirred, under the protection of nitrogen, add SM2 (64.1g, 1.5eq), SM1 (50g, 1.0eq), dichloromethane (500ml, 10V), put it in a low temperature tank and stir to cool down to -5°C, Dropwise addition of DIPEA (45.3g, 2.5eq) was started and the solution gradually cleared. Slowly add TMSCl (22.8g, 1.5eq), keep the internal temperature between -5°C and 5°C, stir the reaction for 2-3h, monitor the end of the reaction, keep cooling down to -30°C to -40°C, add TiCl dropwise 4 (29.2g, 1.1eq), after the dropwise addition was completed, the current temperature was maintained for 1 hour, and the reaction was completed by liquid phase monitoring. Add dichloromethane (350ml / 350ml) dropwise at the current temperature, control the exothermic temperature below -30°C, stir for 2h, then add NaHSO 3 (250ml) solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com