Alpha, beta-unsaturated cyclohexanone derivatives and application thereof
A technology of cyclohexanone and derivatives, which is applied in the field of α,β-unsaturated cyclohexanone derivatives, can solve the problems of consuming large resources and money, and achieve good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
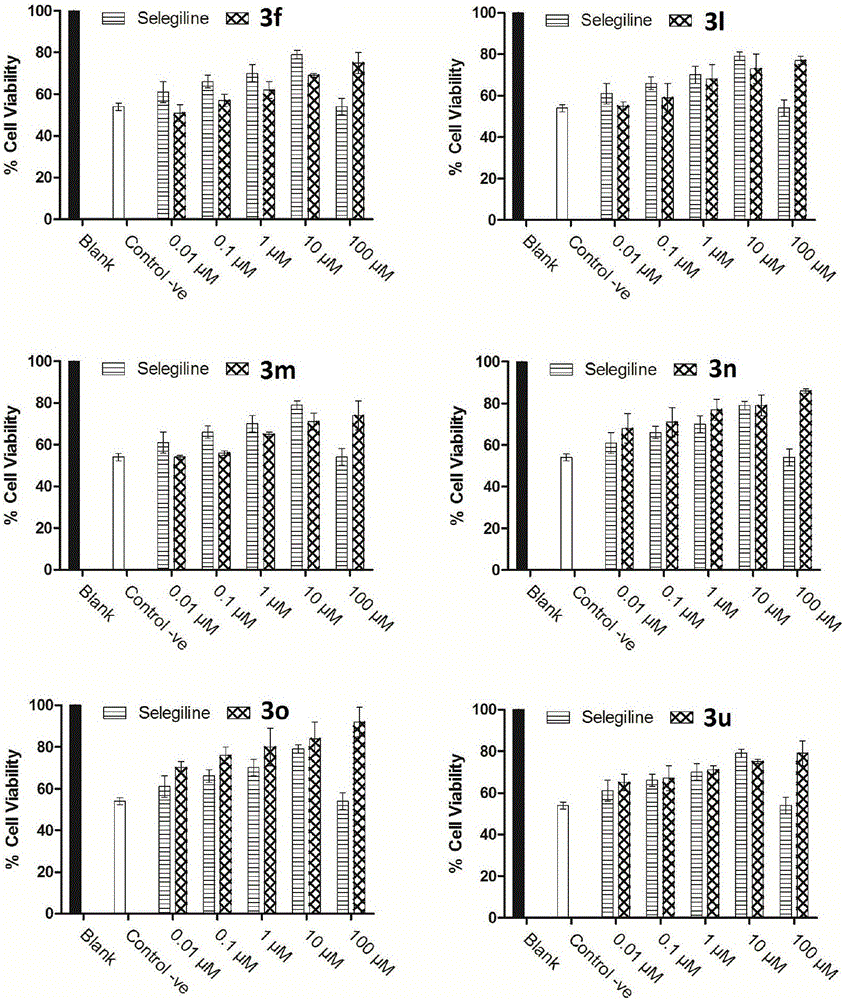
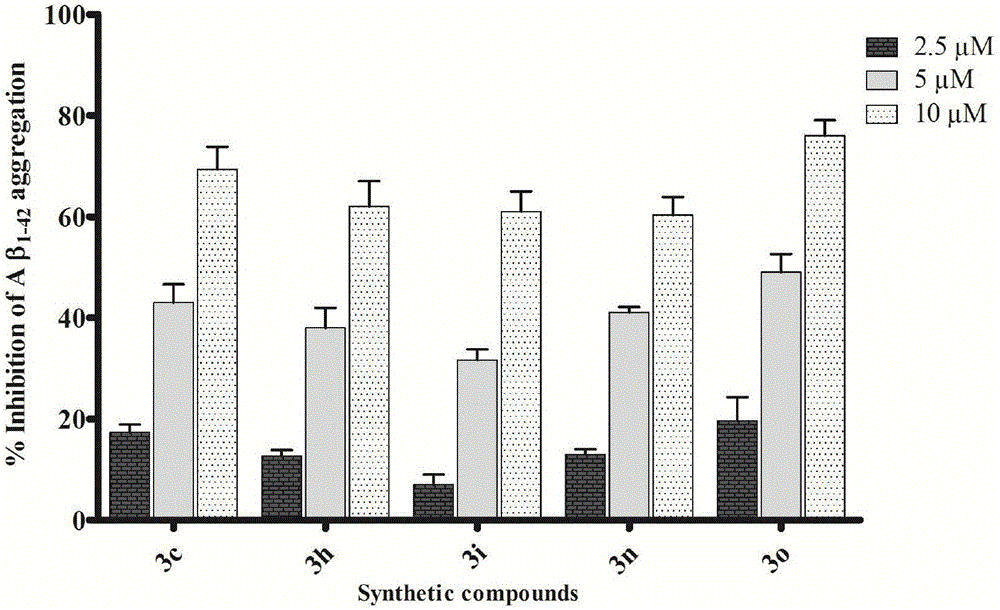
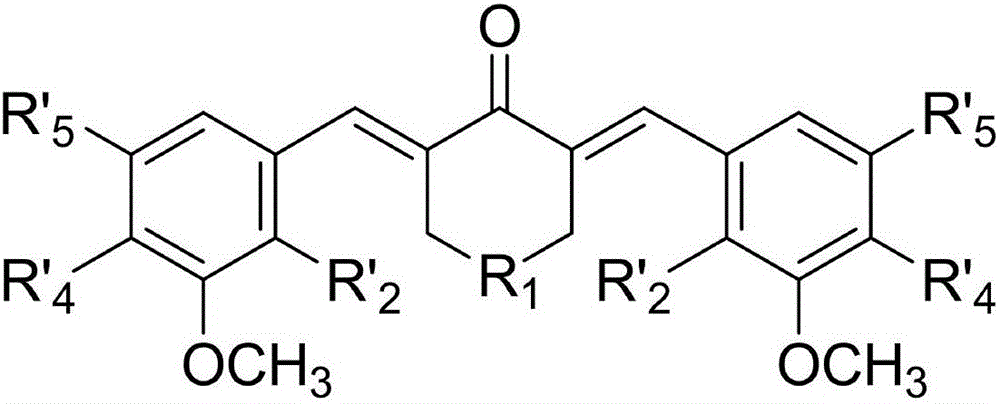
Image
Examples
Embodiment 1
[0057] Synthesis of compound 2,6-bis(2-chloro-3-methoxybenzylidene)cyclohexanone (3a):
[0058] Cyclohexanone (10mmol, 1eq) and 2-chloro-3-methoxybenzaldehyde (20mmol, 2eq) were added to a round bottom flask containing 25mL of ethanol, and the temperature was maintained at 5°C. Afterwards, 40% sodium hydroxide ethanol solution was added. The mixture was stirred at 27°C for 1-24h. The appearance and color change of the precipitate indicated product formation. The reaction was monitored by TLC. When the reaction was complete, acidified ice was added to quench the reaction. Recrystallization or column chromatography was then performed to obtain a purified product with a yield of 85%.
[0059] Mp: 112-114°C; 1 H NMR (500MHz, CDCl 3 )δ: 7.89(s, 2H), 6.90(t, J=8.5Hz, 2H), 6.89(d, J=8.5Hz, 2H), 6.50(d, J=8.5Hz, 2H), 3.72(s, 6H), 2.35(t, J=8.0Hz, 4H), 1.81(m, 2H); 13 C NMR (500MHz, CDCl 3 )δ: 185.2, 152.4, 146.7, 145.2, 144.1, 135.6, 131.2, 107.2, 98.2, 56.7, 28.2, 27.1; HRMS...
Embodiment 2
[0061] 2,6-bis(2-chloro-3,4-dimethoxybenzylidene)cyclohexanone (3b)
[0062] The starting materials are cyclohexanone (10mmol, 1eq) and 2-chloro-3,4-dimethoxybenzaldehyde (20mmol, 2eq). Others are the same as in Example 1, and the yield is 82%.
[0063] Mp:117-118℃; 1 H NMR (500MHz, CDCl 3 )δ: 7.79(s, 2H), 6.92(d, J=8Hz, 2H), 6.54(d, J=8Hz, 2H), 3.70(s, 12H), 2.36(t, J=8.0Hz, 4H) ,1.86(m,2H); 13 C NMR (500MHz, CDCl 3 )δ: 186.2, 150.8, 146.4, 144.5, 142.6, 136.2, 130.1, 108.7, 98.3, 56.6, 56.1, 28.7, 26.9; HRMS (ESI) m / z: 464.35 [M+H] + ,Microanalysis calculated for C 24 h 24 Cl 2 o 5 (463.35), C:62.21%, H:5.22%. Found C:62.25%, H:5.25%.
Embodiment 3
[0065] 2,6-bis(2-bromo-3,4,5-trimethoxybenzylidene)cyclohexanone (3c)
[0066] The starting materials are cyclohexanone (10mmol, 1eq) and 2-bromo-3,4,5-trimethoxybenzaldehyde (20mmol, 2eq). Others are the same as in Example 1, and the yield is 74%.
[0067] Mp: 102-103°C; 1 H NMR (500MHz, CDCl 3 )δ: 7.82(s, 2H), 6.89(s, 2H), 3.75(s, 18H), 2.32(t, J=8.0Hz, 4H), 1.82(m, 2H); 13 C NMR (500MHz, CDCl 3 )δ: 185.4, 151.2, 146.5, 144.2, 144.9, 135.9, 130.5, 108.4, 98.6, 56.5, 56.1, 55.5, 28.5, 27.3; HRMS (ESI) m / z: 613.32 [M+H] + ,Microanalysis calculated for C 26 h 28 Br 2 o 7 (612.30),C:51.00%,H:4.61%.Found C:51.22%,H:4.59%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com