Diaryl macrocycles as modulators of protein kinases
A bicyclic aryl and bicyclic heteroaryl technology, applied in the field of diaryl macrocycle derivatives, can solve problems such as the incomplete understanding of the normal function of human ROS1 kinase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
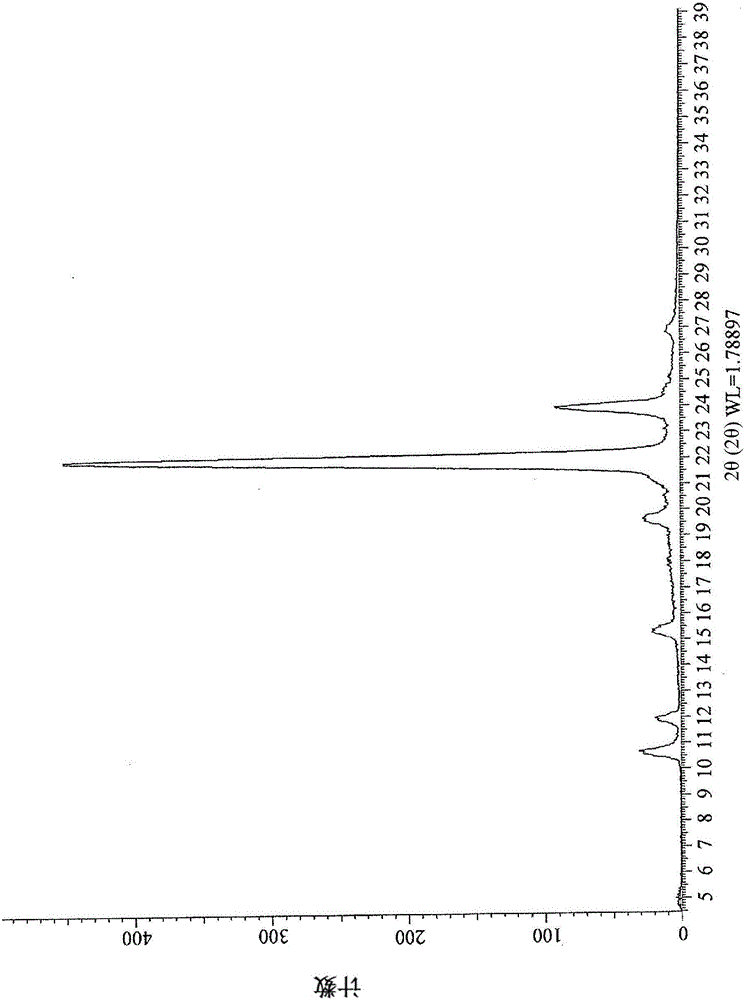
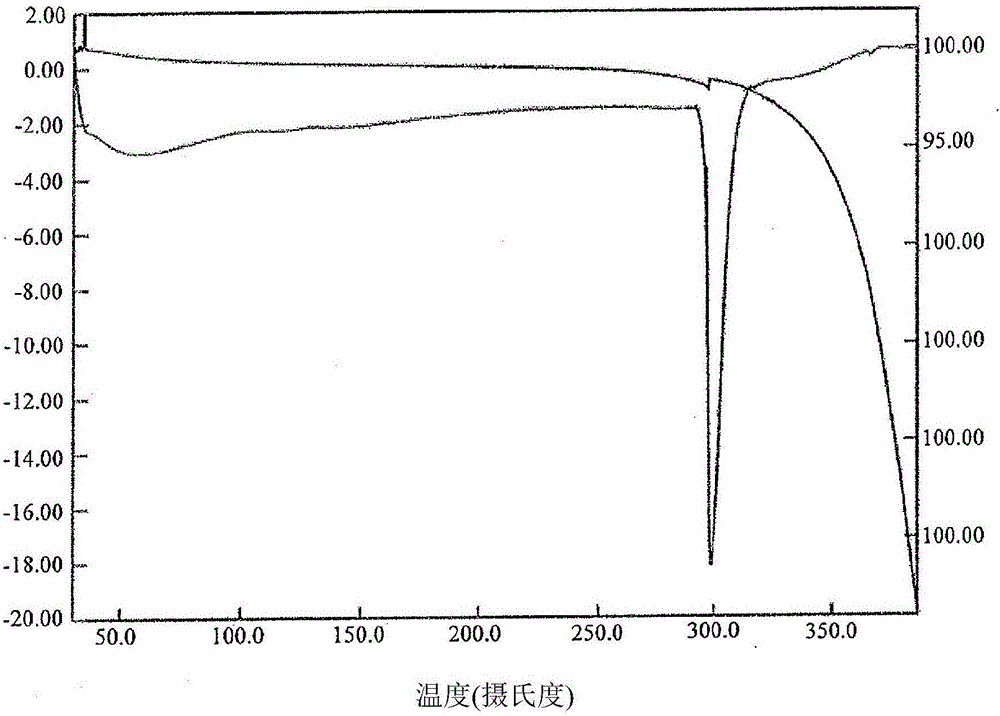
Image
Examples
example
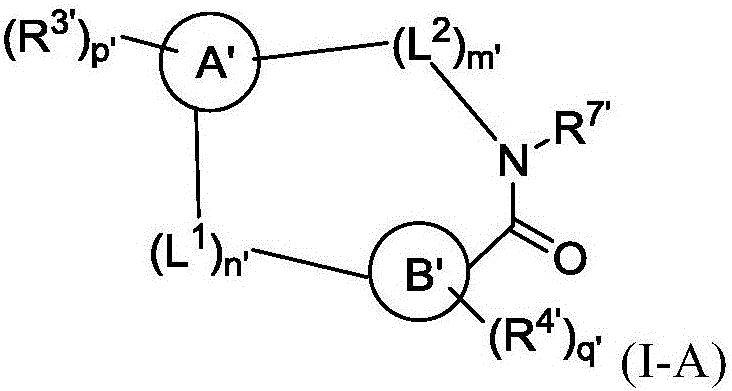
[0378] The following examples are offered to illustrate, not limit, the invention. Those skilled in the art will recognize that the following synthetic reactions and schemes can be modified to obtain other compounds of formula (I) or (I-A) by selection of appropriate starting materials and reagents. Bicyclic heteroaromatics with suitable functionality for use in synthetic methods are commercially available.
[0379] abbreviation The examples described herein use a variety of materials including, but not limited to, those described by the following abbreviations known to those skilled in the art:
[0380]
[0381]
example A6
[0383]
[0384] Step 1. At 16°C in N 2 To a solution of 5-fluoro-2-hydroxybenzaldehyde (500.00 mg, 3.57 mmol, 1.00 eq.) in MeOH (20.00 mL) was added 1-methylpyrrolidin-3-amine (357.43 mg, 3.57 mmol) in one portion ,1.00eq.). The mixture was heated at 16 °C under N 2 Stirring was continued for 10 hours. Then add NaBH 4 (270.00mg, 7.14mmol, 2.00eq.) and the mixture was heated under N at 16°C 2 Stirring was continued for 6 hours. TLC (DCM:MeOH=15:1) showed that the reaction was complete. The reaction mixture was concentrated under reduced pressure to remove MeOH. The residue was diluted with water (50 mL) and extracted with DCM (20 mL x 3). The combined organic layers were washed with brine (50 mL), washed with Na 2 SO 4 Dry, filter and concentrate under reduced pressure to obtain A6-5 (350.00 mg, 1.56 mmol, 43.71% yield) as a yellow solid. 1 HNMR (300MHz, DMSO-d 6 )δ6.94(dd, J=2.7,9.3Hz,1H),6.86(dt,J=3.0,8.6Hz,1H),6.67(dd,J=4.7,8.7Hz,1H),3.71(s,2H ),3.24-3.09(m,1H...
example A8
[0387]
[0388] To ethyl 5-chloropyrazolo[1,5-a]pyrimidine-3-carboxylate (1.25 g, 5.54 mmol) and (R)-2-(1-aminoethyl)-4-fluorophenol HCl salt ( (available from NetChem Inc.) in EtOH (15.83 mL) was added Schoenig's base (3.58 g, 27.70 mmol) and heated to 70 °C for 1.5 hours. The reaction was rotary evaporated to dryness, suspended in water, and extracted with DCM (5 x 50 mL). The combined extracts were washed with Na 2 SO 4 Dry and concentrate under reduced pressure. Flash chromatography (ISCO system, silica (40 g), 0-5% methanol in dichloromethane) provided A8 (1.89 g, 5.49 mmol, 99% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com