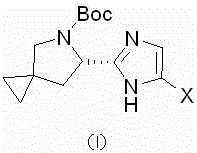
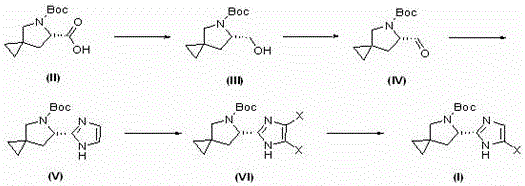
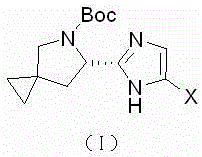
Synthetic method of Ledipasivir intermediate and product thereof
A synthetic method and intermediate technology, which is applied in the field of synthesis of pharmaceutical intermediates, can solve problems such as no synthetic methods, achieve good application prospects and market potential, be conducive to industrial production, and have mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065]
[0066] (1) Synthesis of intermediate product (III)
[0067] Add 48.2 g of compound (II) and 480 mL of tetrahydrofuran into a 1.0 L one-necked bottle, and after cooling down to -4 °C, slowly add 40 mL of borane dimethyl sulfide (10 M ); after the dropwise addition, the temperature was naturally raised to 23°C for 3.5 hours; the sample was sent to LC-MS for detection, and it was found that the starting material was not completely reacted; therefore, the temperature was lowered to 0°C again, and 15 mL of borane dimethyl sulfide was added dropwise (10M), then heated to 24°C and stirred for 1 hour; samples were taken and sent to LC-MS for detection, and the reaction was found to be complete. Cool the reaction solution to -4°C, and slowly add 200 mL of methanol dropwise; after the dropwise addition, stir for 15 minutes, then concentrate to dryness under reduced pressure; then, add 250 mL of dichloromethane and 100 mL of water and stir, the pH of the system = 5~6; then u...
Embodiment 2
[0083]
[0084] (1) Synthesis of intermediate product (III)
[0085] Add 48.2 g of compound (II) and 500 mL of ethyl acetate into a 1.0 L one-necked bottle, and after cooling down to -4 °C, slowly add 40 mL of borane dimethyl sulfide ( 10 M); after the dropwise addition, the temperature was naturally raised to 23°C for 3 hours; the sample was sent to LC-MS for detection, and it was found that the starting material was not completely reacted; therefore, the temperature was lowered to 0°C again, and 15 mL of borane dimethyl was added dropwise thioether (10M), then heated to 25°C and stirred for 1 hour; sampling was sent to LC-MS for detection, and the reaction was found to be complete. Cool the reaction solution to -4°C, and slowly add 200 mL of methanol dropwise; after the dropwise addition, stir for 15 minutes, then concentrate to dryness under reduced pressure; then, add 250 mL of dichloromethane and 100 mL of water and stir. = 5~6; then use saturated sodium bicarbonate s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com