Fleroxacin aldehyde acetal 4-aryl thiosemicarbazide derivatives and its preparation method and application
A technology of arylamino and fleroxacin, which is applied in drug combination, organic chemistry, antineoplastic drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
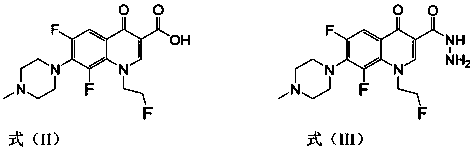
[0032] 1-(2-fluoroethyl)-6,8-difluoro-7-(4-methyl-piperazin-1-yl)-quinolin-4(1H)-one-3-aldehyde 4-benzeneacetal Thiosemicarbazide (I-1), its chemical structural formula is:
[0033]
[0034] That is, the Ar substituent in formula (I) is a benzene ring.
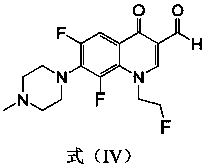
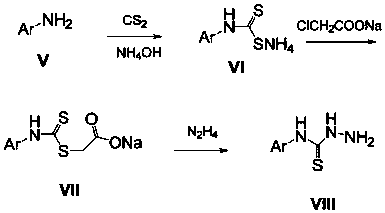
[0035] The preparation method of this compound is: the fleroxacin C-3 aldehyde crude product (1.0g) shown in formula (IV) is dissolved in dehydrated alcohol (30 milliliters), adds the 4-phenylthiosemicarbazide shown in formula (VIII) (0.6g, 3.6mmol), reflux reaction for 10 hours, filtered while hot, washed with ethanol twice, the solid was washed twice with distilled water, dried, and recrystallized with a mixed solvent of DMF-ethanol (V:V=5:3), The light yellow crystal formula (I-1) was obtained, and 0.68 g of the product was obtained, m.p.246-248°C. 1 H NMR (400MHz, DMSO-d 6 ): 11.82(s, 1H, CH=N), 10.05(s, 1H, NH), 8.97(s, 1H, 2-H), 8.46(s, 1H, NH), 7.96~7.48(m, 6H, Ph-H and 5-H),4.87(t,2H,FCH 2 ),4.68(t,2H,NCH 2 ),...
Embodiment 2
[0037] 1-(2-fluoroethyl)-6,8-difluoro-7-(4-methyl-piperazin-1-yl)-quinolin-4(1H)-one-3-aldehyde acetal 4-( 4-methylphenyl) thiosemicarbazide (I-2), its chemical structural formula is:
[0038]
[0039] That is, Ar in formula (I) is 4-methylphenyl.
[0040] The preparation method of this compound is: the fleroxacin C-3 aldehyde crude product (1.0g) shown in formula (IV) is dissolved in dehydrated alcohol (30 milliliters), adds the 4-(4-methyl aldehyde shown in formula (VIII) Phenyl)thiosemicarbazide (0.6g, 3.3mmol), reflux reaction for 12 hours, filtered while hot, the solid was washed twice with ethanol and distilled water twice, dried, and washed with DMF-ethanol (V:V=5:3 ) mixed solvent for recrystallization to obtain light yellow crystals of formula (I-2), 0.66 g of the product, m.p.235-237°C. 1 H NMR (400MHz, DMSO-d 6 ): 11.78(s, 1H, CH=N), 9.96(s, 1H, NH), 8.95(s, 1H, 2-H), 8.46(s, 1H, NH), 7.83~7.46(m, 5H, Ph-H and 5-H),4.86(t,2H,FCH 2 ),4.68(t,2H,NCH 2 ), 3.35 ...
Embodiment 3
[0042] 1-(2-fluoroethyl)-6,8-difluoro-7-(4-methyl-piperazin-1-yl)-quinolin-4(1H)-one-3-aldehyde acetal 4-( 4-methoxyphenyl) thiosemicarbazide (I-3), its chemical structural formula is:
[0043]
[0044] That is, Ar in formula (I) is 4-methoxyphenyl.
[0045] The preparation method of this compound is: the fleroxacin C-3 aldehyde crude product (1.0g) shown in formula (IV) is dissolved in dehydrated alcohol (30 milliliters), adds the 4-(4-methoxy compound shown in formula (VIII) phenyl) thiosemicarbazide (0.7g, 3.6mmol), reflux reaction for 8 hours, filtered while hot, the solid was washed twice with ethanol, washed twice with distilled water, dried, and washed with DMF-ethanol (V:V=5: 3) Recrystallization from the mixed solvent to obtain the pale yellow crystal formula (I-3), 0.76 g of the product, m.p.>250°C. 1 H NMR (400MHz, DMSO-d 6 ): 11.83(s, 1H, CH=N), 10.12(s, 1H, NH), 9.15(s, 1H, 2-H), 8.50(s, 1H, NH), 8.15~7.52(m, 5H, Ph-H and 5-H),4.88(t,2H,FCH 2 ),4.68(t,2H,N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com