A class of thiaevodipine compounds and their application in antitumor drugs
An anti-tumor drug, a technology of hybrid evodiam, applied in the field of medicine, can solve the problems of low anti-tumor activity, poor selectivity of evodial derivatives, normal cytotoxicity that cannot be ignored, etc., and achieves the effect of good activity and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
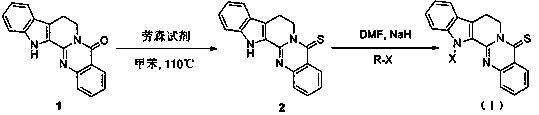
[0025] Example 1: Synthesis of compound 2 (thioevodiamine)
[0026] In a three-necked flask equipped with a thermometer and a condenser tube, add evodiamine (0.50 g, 3.50 mmol), Lawson’s reagent (2.12 g, 5.25 mmol), toluene (100 mL), and stir the reaction at 110 °C for 6 h. Thin-layer chromatography (TLC), the developer is petroleum ether (PE): ethyl acetate (EtOAc) = 5:1) to monitor the reaction. After the reaction, the solvent was removed by rotary evaporation, and the product was separated and purified by silica gel column chromatography (elution condition PE:EtOAc=2:1). The product is yellow crystals, 0.29 g, yield: 54.5%, product purity: 99.9%; m.p. 216-218 ℃, TLC developer: PE : EtOAc = 5:1, product Rf value 0.52, separated and purified by silica gel column chromatography , Elution condition PE: EtOAc= 2:1; FT-IR: 3445( ν C-H ,Ar-H), 3051 ( υ C-H , CH 3 ), 2920( υ C-H , CH 2 ), 2898 ( υ C-H , CH), 1617 ( υ C=O , N-C=O),1595( υ C=C ), 1471( δ C-H ,...
Embodiment 2
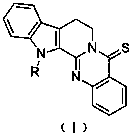
[0027] Embodiment 2: the synthesis of compound Ia
[0028] In a three-necked flask equipped with a thermometer and a condenser tube, add thioevodiamine (0.20 g, 0.66 mmol), NaH (20.59 mg, 0.86 mmol), DMF (10 mL, 4Å molecular sieve to remove water), and stir at room temperature for 20 min, slowly added halogenated hydrocarbon (0.99 mmol). The reaction was stirred at 50 °C and monitored by TLC. Post-treatment after the reaction: add water (10 mL) to quench the reaction, extract with ethyl acetate (20 mL) three times, combine the organic phases, wash twice with saturated brine, anhydrous Na 2 SO 4 Dry for 2 h, filter with suction, remove the solvent by rotary evaporation, and separate and purify by silica gel column chromatography to obtain the product. Yellow crystal, 0.20g, yield: 87.6%, product purity: 99.9%; m.p. 163-165 ℃; TLC developer is PE: EtOAc = 1:1, product Rf value is 0.24, separated and purified by silica gel column chromatography, washed Deconditioning PE: EtOA...
Embodiment 3
[0029] Embodiment 3: the synthesis of compound Ib
[0030] In a three-necked flask equipped with a thermometer and a condenser tube, add thioevodiamine (0.20 g, 0.66 mmol), NaH (20.59 mg, 0.86 mmol), DMF (10 mL, 4Å molecular sieve to remove water), and stir at room temperature for 20 min, slowly added halogenated hydrocarbon (0.99 mmol). The reaction was stirred at 50 °C and monitored by TLC. Post-treatment after the reaction: add water (10 mL) to quench the reaction, extract with ethyl acetate (20 mL) three times, combine the organic phases, wash twice with saturated brine, anhydrous Na 2 SO 4 Dry for 2 h, filter with suction, remove the solvent by rotary evaporation, and separate and purify by silica gel column chromatography to obtain the product. Yellow crystal, 0.18g, yield: 78.8%, product purity: 98.5%; m.p. 176-178 ℃; TLC developer is PE: EtOAc=5:1, product Rf value is 0.45, silica gel column chromatography, elution conditions : PE:EtOAc = 20:1; FT-IR: 3138 ( υ C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com